An Electrophilic Bromine Redox Catalysis for the Synthesis of Indole Alkaloid Building Blocks by Selective Aliphatic C−H Amination
Dr. Julien Bergès
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorDr. Belén García
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Kilian Muñiz
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
ICREA, Pg. Lluís Companys 23, 08010 Barcelona, Spain
Search for more papers by this authorDr. Julien Bergès
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorDr. Belén García
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Kilian Muñiz
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
ICREA, Pg. Lluís Companys 23, 08010 Barcelona, Spain
Search for more papers by this authorGraphical Abstract
Abstract
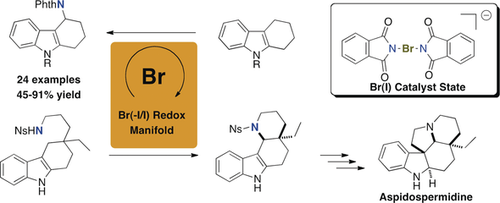
A new homogeneous bromine(−I/I) redox catalysis is described, which is based on monomeric bromine(I) compounds containing transferable phthalimidato groups. These catalysts enable intermolecular C−H amination reactions at previously unaccessible aliphatic positions and thus enlarge the synthetic potential of direct C−N bond formation, including its application in the synthesis of alkaloid building blocks. This aspect is demonstrated by a new synthetic approach to aspidospermidine. In addition to the development of the catalyst system, the structures of the involved bromine(I) key catalysts were fully elucidated, including by X-ray analyses.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201808939-sup-0001-misc_information.pdf10.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. E. Saxton in Chemistry of Heterocyclic Compounds, Wiley, New York, 2008, pp. 331–437;
- 1bM. Toyota, M. Ihara, Nat. Prod. Rep. 1998, 15, 327–340;
- 1cW. A. Creasey in Monoterpenoid Indole Alkaloids, Supplement to Vol. 25, Part 4 (Ed.: ), Wiley, New York, 1994, pp. 715–754.
- 2
- 2aJ. Bonjoch, D. Sole, Chem. Rev. 2000, 100, 3455;
- 2bJ. S. Cannon, L. E. Overman, Angew. Chem. Int. Ed. 2012, 51, 4288; Angew. Chem. 2012, 124, 4362.
- 3K. W. Fiori, J. Du Bois, J. Am. Chem. Soc. 2007, 129, 562.
- 4
- 4aE. Fischer, F. Jourdan, Ber. Dtsch. Chem. Ges. 1883, 16, 2241;
10.1002/cber.188301602141 Google Scholar
- 4bB. Robinson, Chem. Rev. 1963, 63, 373.
- 5A. Molnár, G. A. Olah, G. K. S. Prakash, in Hydrocarbon Chemistry, Wiley, New York, 2017, Chap. 9.
10.1002/9781119390541 Google Scholar
- 6Reviews:
- 6aS. Minakata, Acc. Chem. Res. 2009, 42, 1172;
- 6bK. Muñiz, Pure Appl. Chem. 2013, 85, 755.
- 7
- 7aJ. U. Jeong, B. Tao, I. Sagasser, H. Hennigen, K. B. Sharpless, J. Am. Chem. Soc. 1998, 120, 6844;
- 7bP. Dauban, R. H. Dodd, Tetrahedron Lett. 2001, 42, 1037;
- 7cV. V. Thakur, A. Sudalai, Tetrahedron Lett. 2003, 44, 989;
- 7dS. C. Coote, P. O'Brian, A. C. Whitwood, Org. Biomol. Chem. 2008, 6, 4299;
- 7eP. Chávez, J. Kirsch, C. H. Hövelmann, J. Streuff, M. Martínez-Belmonte, E. C. Escudero-Adán, E. Martin, K. Muñiz, Chem. Sci. 2012, 3, 2375;
- 7fP. Becker, T. Duhamel, C. Martínez, K. Muñiz, Angew. Chem. Int. Ed. 2018, 57, 5166; Angew. Chem. 2018, 130, 5262.
- 8
- 8aJ. A. Souto, C. Martínez, I. Velilla, K. Muñiz, Angew. Chem. Int. Ed. 2013, 52, 1324; Angew. Chem. 2013, 125, 1363;
- 8bK. Muñiz, Acc. Chem. Res. 2018, 57, 1507.
- 9
- 9aS. Gabriel, Chem. Ber. 1887, 20, 2224;
10.1002/cber.18870200227 Google Scholar
- 9bO. Mitsunobu, Comput. Org. Synth. 1991, 6, 79.
- 10
- 10aK. Kiyokawa, S. Yahata, T. Kojima, S. Minakata, Org. Lett. 2014, 16, 4646;
- 10bL. Marchetti, A. Kantak, R. Davis, B. DeBoef, Org. Lett. 2015, 17, 358;
- 10cA. A. Kantak, L. Marchetti, B. DeBoef, Chem. Commun. 2015, 51, 3574;
- 10dK. Moriyama, K. Ishida, H. Togo, Org. Lett. 2012, 14, 946; for derivatives as putative intermediates in arene amination, see
- 10eA. A. Kantak, S. Potavathri, R. A. Barham, K. M. Romano, B. DeBoef, J. Am. Chem. Soc. 2011, 133, 19960;
- 10fH. J. Kim, J. Kim, S. H. Cho, S. Chang, J. Am. Chem. Soc. 2011, 133, 16382.
- 11L. Hadjiarapoglou, S. Spyroudis, A. Varvoglis, Synthesis 1983, 207.
- 12K. Kiyokawa, T. Kosaka, T. Kojima, S. Minakata, Angew. Chem. Int. Ed. 2015, 54, 13719–13723; Angew. Chem. 2015, 127, 13923–13927.
- 13See the Supporting Information for details.
- 14X-ray crystallographic data for compounds 1, 4 a, 7, 9 a, 9 b, 13, and 17 have been deposited with the Cambridge Crystallographic Data Centre database (http://www.ccdc.cam.ac.uk/) under codes CCDC 1857935 (1), 1857936 (4 a), 1857937 (9 a), 1857938 (9 b), 1857939 (13), and 1857940 (17), respectively.
- 15
- 15aN. Gulzar, M. Klussmann, Org. Biomol. Chem. 2013, 11, 4516;
- 15bX. Liu, Y. Zhou, Z. Yang, Q. Li, L. Zhao, P. Liu, J. Org. Chem. 2018, 83, 4665;
- 15cL. Jiang, X. Xie, L. Zu, RSC Adv. 2015, 5, 9204.
- 16For a previous variant of a stoichiometric iodine-mediated oxygenation reaction without 1/4-regioselectivity, see H. Zaimoku, T. Hatta, T. Taniguchi, H. Ishibashi, Org. Lett. 2012, 14, 6088.
- 17Reviews:
- 17aP. Finkbeiner, B. Nachtsheim, Synthesis 2013, 45, 979;
- 17bM. Uyanik, K. Ishihara, ChemCatChem 2012, 4, 177.
- 18E. Stempel, T. Gaich, Acc. Chem. Res. 2016, 49, 2390.
- 19
- 19aM. Elding, A.-K. Larsson, G. Svenson, J. Albertsson, Acta Crystallogr. Sect. C 1992, 48, 2078;
- 19bJ. E. Barry, M. Finkelstein, W. M. Moore, S. D. Ross, L. Ebertson, Tetrahedron Lett. 1984, 25, 2847.
- 20For the pioneering synthesis of a bisacetoxy derivative, see
- 20aMd. A. Hashem, A. Jung, M. Ries, A. Kirschning, Synlett 1998, 195; for application in conceptually nonrelated oxidations, see
- 20bH. Monenschein, G. Sourkouni-Argirusi, K. M. Schubothe, T. O'Hare, A. Kirschning, Org. Lett. 1999, 1, 2101;
- 20cA. Kirschning, G. Sourkouni-Argirusi, M. Brünjes, Adv. Synth. Catal. 2003, 345, 635;
- 20dK. Kloth, M. Brünjes, E. Kunst, F. Gallier, A. Adibekian, A. Kirschning, Adv. Synth. Catal. 2005, 347, 1423;
- 20eK. C. Nicolaou, P. K. Sasmal, T. V. Koftis, A. Converso, E. Loizidou, F. Kaiser, A. J. Roecker, C. C. Dellios, X.-W. Sun, G. Petrovic, Angew. Chem. Int. Ed. 2005, 44, 3447; Angew. Chem. 2005, 117, 3513;
- 20fP. Pasetto, R. W. Franck, J. Org. Chem. 2003, 68, 8042.
- 21
- 21aW. Kurosawa, T. Kan, T. Fukuyama, Org. Synth. 2002, 79, 186;
- 21bA. Fujiwara, T. Kan, T. Fukuyama, Synlett 2000, 1667.
- 22
- 22aM. A. Toczko, C. H. Heathcock, J. Org. Chem. 2000, 65, 2642;
- 22bZ. Li, S. Zhang, S. Wu, X. Shen, L. Zou, F. Wang, X. Li, F. Peng, H. Zhang, Z. Shao, Angew. Chem. Int. Ed. 2013, 52, 4117; Angew. Chem. 2013, 125, 4211;
- 22cJ.-Y. Kim, C.-H. Suhl, J.-H. Lee, C.-G. Cho, Org. Lett. 2017, 19, 6168.
- 23
- 23aP. J. Gritsch, C. Leitner, M. Pfaffenbach, T. Gaich, Angew. Chem. Int. Ed. 2014, 53, 1208; Angew. Chem. 2014, 126, 1230;
- 23bJ. Kalepu, P. Gandeepan, L. Ackermann, L. T. Pilarski, Chem. Soc. Rev. 2018, 9, 4203;
- 23cJ. Bosch, M. Amat, E. Sanfeliu, Tetrahedron 1985, 41, 2557.
- 24T. Bach, J. P. Hehn, Angew. Chem. Int. Ed. 2011, 50, 1000; Angew. Chem. 2011, 123, 1032.