Single-Electron Transfer Reactions in Frustrated and Conventional Silylium Ion/Phosphane Lewis Pairs
M. Sc. Anastasia Merk
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
Search for more papers by this authorDr. Henning Großekappenberg
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
Search for more papers by this authorDr. Marc Schmidtmann
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
Search for more papers by this authorM. Sc. Marcel-Philip Luecke
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorM. Sc. Christian Lorent
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorProf. Dr. Matthias Driess
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorProf. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorCorresponding Author
Dr. Hendrik F. T. Klare
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas Müller
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
Search for more papers by this authorM. Sc. Anastasia Merk
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
Search for more papers by this authorDr. Henning Großekappenberg
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
Search for more papers by this authorDr. Marc Schmidtmann
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
Search for more papers by this authorM. Sc. Marcel-Philip Luecke
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorM. Sc. Christian Lorent
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorProf. Dr. Matthias Driess
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorProf. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorCorresponding Author
Dr. Hendrik F. T. Klare
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas Müller
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
Search for more papers by this authorDedicated to Professor Werner Uhl on the occasion of his 65th birthday
Graphical Abstract
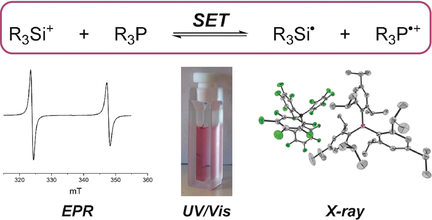
An electron against frustration! Silylium ions accept an electron from sterically hindered triarylphosphanes, regardless of whether these Lewis acids form a frustrated or a classical Lewis pair with the phosphane Lewis base. The thus-generated phosphoniumyl radical cations were characterized by EPR and UV/Vis absorption spectroscopy and single-crystal X-ray diffraction (see picture).
Abstract
Silylium ions undergo a single-electron reduction with phosphanes, leading to transient silyl radicals and the corresponding stable phosphoniumyl radical cations. As supported by DFT calculations, phosphanes with electron-rich 2,6-disubstituted aryl groups are sufficiently strong reductants to facilitate this single-electron transfer (SET). Frustration as found in kinetically stabilized triarylsilylium ion/phosphane Lewis pairs is not essential, and silylphosphonium ions, which are generated by conventional Lewis adduct formation of solvent-stabilized trialkylsilylium ions and phosphanes, engage in the same radical mechanism. The trityl cation, a Lewis acid with a higher electron affinity, even oxidizes trialkylphosphanes, such as tBu3P, which does not react with either B(C6F5)3 or silylium ions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201808922-sup-0001-misc_information.pdf2.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent overviews of frustrated Lewis pair chemistry, see:
- 1aD. W. Stephan, Science 2016, 354, 1248;
- 1bD. W. Stephan, J. Am. Chem. Soc. 2015, 137, 10018–10032;
- 1cD. W. Stephan, Acc. Chem. Res. 2015, 48, 306–316;
- 1dD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400–6441; Angew. Chem. 2015, 127, 6498–6541.
- 2For mechanistic studies of FLP reactivity, see:
- 2aT. A. Rokob, A. Hamza, A. Stirling, T. Soós, I. Pápai, Angew. Chem. Int. Ed. 2008, 47, 2435–2438; Angew. Chem. 2008, 120, 2469–2472;
- 2bA. Stirling, A. Hamza, T. A. Rokob, I. Pápai, Chem. Commun. 2008, 3148–3150;
- 2cY. Guo, S. Li, Inorg. Chem. 2008, 47, 6212–6219;
- 2dT. A. Rokob, A. Hamza, A. Stirling, I. Pápai, J. Am. Chem. Soc. 2009, 131, 2029–2036;
- 2eT. A. Rokob, A. Hamza, I. Pápai, J. Am. Chem. Soc. 2009, 131, 10701–10710;
- 2fA. Hamza, A. Stirling, T. A. Rokob, I. Pápai, Int. J. Quantum Chem. 2009, 109, 2416–2425;
- 2gS. Grimme, H. Kruse, L. Goerigk, G. Erker, Angew. Chem. Int. Ed. 2010, 49, 1402–1405; Angew. Chem. 2010, 122, 1444–1447;
- 2hG. Erős, H. Mehdi, I. Pápai, T. A. Rokob, P. Király, G. Tárkányi, T. Soós, Angew. Chem. Int. Ed. 2010, 49, 6559–6563; Angew. Chem. 2010, 122, 6709–6713;
- 2iB. Schirmer, S. Grimme, Chem. Commun. 2010, 46, 7942–7944;
- 2jI. Bakó, A. Stirling, S. Bálint, I. Pápai, Dalton Trans. 2012, 41, 9023–9025;
- 2kD. M. Camaioni, B. Ginovska-Pangovska, G. K. Schenter, S. M. Kathmann, T. Autrey, J. Phys. Chem. A 2012, 116, 7228–7237;
- 2lT. A. Rokob, I. Bakó, A. Stirling, A. Hamza, I. Pápai, J. Am. Chem. Soc. 2013, 135, 4425–4437;
- 2mL. L. Zeonjuk, N. Vankova, A. Mavrandonakis, T. Heine, G.-V. Röschenthaler, J. Eicher, Chem. Eur. J. 2013, 19, 17413–17424;
- 2nH. Zaher, A. E. Ashley, M. Irwin, A. L. Thompson, M. J. Gutmann, T. Krämer, D. O'Hare, Chem. Commun. 2013, 49, 9755–9757;
- 2oL. Rocchigiani, G. Ciancaleoni, C. Zuccaccia, A. Macchioni, J. Am. Chem. Soc. 2014, 136, 112–115;
- 2pM. Pu, T. Privalov, ChemPhysChem 2014, 15, 2936–2944;
- 2qM. Pu, T. Privalov, ChemPhysChem 2014, 15, 3714–3719;
- 2rM. Pu, T. Privalov, Inorg. Chem. 2014, 53, 4598–4609;
- 2sM. Pu, T. Privalov, Isr. J. Chem. 2015, 55, 179–195;
- 2tC. Bannwarth, A. Hansen, S. Grimme, Isr. J. Chem. 2015, 55, 235–242;
- 2uL. Liu, B. Lukose, B. Ensing, J. Phys. Chem. C 2017, 121, 2046–2051;
- 2vA. Y. Houghton, T. Autrey, J. Phys. Chem. A 2017, 121, 8785–8790.
- 3
- 3aL. L. Liu, L. L. Cao, Y. Shao, G. Ménard, D. W. Stephan, Chem 2017, 3, 259–267;
- 3bL. L. Liu, L. L. Cao, D. Zhu, J. Zhou, D. W. Stephan, Chem. Commun. 2018, 54, 7431–7434; for earlier reports indicating a radical mechanism, see:
- 3cG. C. Welch, D. W. Stephan, J. Am. Chem. Soc. 2007, 129, 1880–1881;
- 3dW. E. Piers, A. J. V. Marwitz, L. G. Mercier, Inorg. Chem. 2011, 50, 12252–12262.
- 4For silylium ion/phosphane Lewis pair chemistry, see:
- 4aA. Schäfer, M. Reißmann, A. Schäfer, W. Saak, D. Haase, T. Müller, Angew. Chem. Int. Ed. 2011, 50, 12636–12638; Angew. Chem. 2011, 123, 12845–12848;
- 4bM. Reißmann, A. Schäfer, S. Jung, T. Müller, Organometallics 2013, 32, 6736–6744;
- 4cT. J. Herrington, B. J. Ward, L. R. Doyle, J. McDermott, A. J. P. White, P. A. Hunt, A. E. Ashley, Chem. Commun. 2014, 50, 12753–12756; for the solid-state structure and Lewis acidity of 1 a, see:
- 4dA. Schäfer, M. Reißmann, S. Jung, A. Schäfer, W. Saak, E. Brendler, T. Müller, Organometallics 2013, 32, 4713–4722;
- 4eH. Großekappenberg, M. Reißmann, M. Schmidtmann, T. Müller, Organometallics 2015, 34, 3756–3763; see also
- 4fR. K. Schmidt, K. Müther, C. Mück-Lichtenfeldt, S. Grimme, M. Oestreich, J. Am. Chem. Soc. 2012, 134, 4421–4428.
- 5For silylium ion/silylene Lewis pair chemistry, see:
- 5aA. Schäfer, A. Schäfer, T. Müller, Dalton Trans. 2010, 39, 9296–9303;
- 5bA. Schäfer, M. Reißmann, A. Schäfer, M. Schmidtmann, T. Müller, Chem. Eur. J. 2014, 20, 9381–9386.
- 6For silylium ion/N-heterocyclic carbene Lewis pair chemistry, see: M. F. Silva Valverde, E. Theuergarten, T. Bannenberg, M. Freytag, P. G. Jones, M. Tamm, Dalton Trans. 2015, 44, 9400–9408.
- 7For general Reviews on silylium ion chemistry, see:
- 7aV. Ya. Lee, A. Sekiguchi in Organosilicon Compounds, Vol. 1 (Ed.: ), Academic Press, Oxford, 2017, pp. 197–230;
- 7bT. Müller in Structure and Bonding, Vol. 155 (Ed.: ), Springer, Berlin, 2014, pp. 107–162;
- 7cT. Müller in Science of Synthesis: Knowledge Updates 2013/3 (Ed.: ), Thieme, Stuttgart, 2013, pp. 1–42;
- 7dH. F. T. Klare, M. Oestreich, Dalton Trans. 2010, 39, 9176–9184.
- 8S. Sasaki, K. Sutoh, F. Murakami, M. Yoshifuji, J. Am. Chem. Soc. 2002, 124, 14830–14831.
- 9For the molecular structure of [Tipp3P⋅][Al(OC(CF3)3)4], see: X. Pan, X. Chen, T. Li, Y. Li, X. Wang, J. Am. Chem. Soc. 2013, 135, 3414–3417.
- 10For triarylphosphoniumyl radical cations, see:
- 10aM. Culcasi, Y. Berchadsky, G. Gronchi, P. Tordo, J. Org. Chem. 1991, 56, 3537–3542;
- 10bR. T. Boeré, A. M. Bond, S. Cronin, N. W. Duffy, P. Hazendonk, J. D. Masuda, K. Pollard, T. L. Roemmele, P. Tran, Y. Zhang, New J. Chem. 2008, 32, 214–231;
- 10cS. Tojo, S. Yasui, M. Fujitsuka, T. Majima, J. Org. Chem. 2006, 71, 8227–8232.
- 11For triarylsilyl radicals, see:
- 11aH. Sakurai, H. Umino, H. Sugiyama, J. Am. Chem. Soc. 1980, 102, 6837–6840;
- 11bM. J. S. Gynane, M. F. Lappert, P. I. Riley, J. Organomet. Chem. 1980, 202, 5–12;
- 11cW. P. Neumann, K.-D. Schultz, R. Vieler, J. Organomet. Chem. 1984, 264, 179–191.
- 12For general Reviews of silyl radicals, see:
- 12aB. Tumanskii, M. Karni, Y. Apeloig in Organosilicon Compounds, Vol. 1 (Ed.: ), Academic Press, Oxford, 2017, pp. 231–294;
- 12bB. Tumanskii, M. Karni, Y. Apeloig in Encyclopedia of Radicals in Chemistry, Biology and Materials, Vol. 4 (Eds.: ), Wiley, Chichester, 2012, pp. 2117–2146;
- 12cC. Chatgilialoglu, Chem. Rev. 1995, 95, 1229–1251.
- 13G. Ménard, J. A. Hatnean, H. J. Cowley, A. J. Lough, J. M. Rawson, D. W. Stephan, J. Am. Chem. Soc. 2013, 135, 6446–6449.
- 14L. Omann, B. Pudasaini, E. Irran, H. F. T. Klare, M.-H. Baik, M. Oestreich, Chem. Sci. 2018, 9, 5600–5607.
- 15For silylphosphonium ions, see:
- 15aD. Chen, V. Leich, F. Pan, J. Klankermayer, Chem. Eur. J. 2012, 18, 5184–5187;
- 15bW. Nie, H. F. T. Klare, M. Oestreich, R. Fröhlich, G. Kehr, G. Erker, Z. Naturforsch. B 2012, 67, 987–994;
- 15cRef. [4b];
- 15dRef. [4c];
- 15eM. H. Holthausen, J. M. Bayne, I. Mallov, R. Dobrovetsky, D. W. Stephan, J. Am. Chem. Soc. 2015, 137, 7298–7301;
- 15fI. Mallov, A. J. Ruddy, H. Zhu, S. Grimme, D. W. Stephan, Chem. Eur. J. 2017, 23, 17692–17696.
- 16For the trityl radical, see:
- 16aM. Gomberg, Ber. Dtsch. Chem. Ges. 1900, 33, 3150–3163;
- 16bM. Gomberg, J. Am. Chem. Soc. 1900, 22, 757–771;
- 16cD. B. Chesnut, G. J. Sloan, J. Chem. Phys. 1960, 33, 637–638;
- 16dA. K. Zarkadis, W. P. Neumann, W. Uzick, Chem. Ber. 1985, 118, 1183–1192;
- 16eW. P. Neumann, W. Uzick, A. K. Zarkadis, J. Am. Chem. Soc. 1986, 108, 3762–3770;
- 16fS. Fukuzumi, T. Kitano, M. Ishikawa, J. Am. Chem. Soc. 1990, 112, 5631–5632.
- 17
- 17aL. Cabrera, G. C. Welch, J. D. Masuda, P. Wei, D. W. Stephan, Inorg. Chim. Acta 2006, 359, 3066–3071;
- 17bE. Follet, P. Mayer, D. S. Stephenson, A. R. Ofial, G. Berionni, Chem. Eur. J. 2017, 23, 7422–7427.