Light-Induced Gold-Catalyzed Hiyama Arylation: A Coupling Access to Biarylboronates
Corresponding Author
Jin Xie
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 P. R. China
Search for more papers by this authorKohei Sekine
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorSina Witzel
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorPetra Krämer
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorMatthias Rudolph
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorFrank Rominger
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorCorresponding Author
A. Stephen K. Hashmi
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589 Saudi Arabia
Search for more papers by this authorCorresponding Author
Jin Xie
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 P. R. China
Search for more papers by this authorKohei Sekine
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorSina Witzel
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorPetra Krämer
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorMatthias Rudolph
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorFrank Rominger
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorCorresponding Author
A. Stephen K. Hashmi
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589 Saudi Arabia
Search for more papers by this authorGraphical Abstract
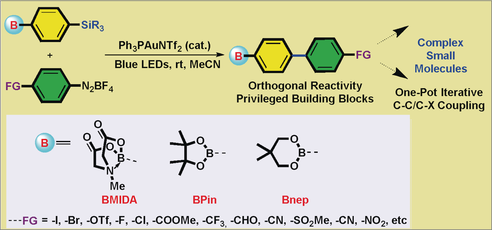
Organoboron compounds are versatile synthetic building blocks. A new strategy, namely a photochemical gold-catalyzed chemoselective Hiyama arylation of B,Si bimetallic reagents with diazonium salts, which is orthogonal to common strategies and therefore a unique tool for synthesis of valuable biarylboronates, has been developed. With this new methodology a wide array of diversely functionalized sp2- and sp3-hybridized biarylboronates were obtained.
Abstract
Organoboron compounds are versatile synthetic building blocks. We herein report a new strategy, a photochemical gold-catalyzed chemo-selective Hiyama arylation of B,Si bifunctionalized reagents with diazonium salts, which is orthogonal to common strategies and therefore a unique tool for synthesis of valuable biarylboronates. With this new methodology a wide array of diversely functionalized sp2- and sp3-hybridized biarylboronates were obtained. Notably, the synergism of gold catalysis with copper catalysis or palladium catalysis, allows for one-pot iterative C−X (heteroatom) and C−C couplings for the rapid assembly of several simple fragments to relatively complex molecules. Mechanistic studies indicated that photosensitizer-free conditions were superior to gold/Ru(bpy)3Cl2 dual catalysis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201806427-sup-0001-misc_information.pdf5.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1I. Cepanec, Synthesis of biaryls, Elsevier, Amsterdam, 2010.
- 2C. Johansson Seechurn, M. O. Kitching, T. J. Colacot, V. Snieckus, Angew. Chem. Int. Ed. 2012, 51, 5062–5085; Angew. Chem. 2012, 124, 5150–5174.
- 3O. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074–1086.
- 4Y. Yang, J. Lan, J. You, Chem. Rev. 2017, 117, 8787–8863.
- 5K. Yoshida, T. H. In, Boronic acids: preparation and applications in organic synthesis and medicine, Wiley-VCH, Weinheim, 2005.
- 6A. J. J. Lennox, G. C. Lioyd-Jones, Chem. Soc. Rev. 2014, 43, 412–443.
- 7E. P. Gillis, M. D. Burke, J. Am. Chem. Soc. 2007, 129, 6716–6717.
- 8J. Li, S. G. Ballmer, E. P. Gillis, S. Fujii, M. J. Schmidt, A. M. E. Palazzolo, J. W. Lehmann, G. F. Morehouse, M. D. Burke, Science 2015, 347, 1221–1226.
- 9H. Noguchi, T. Shioda, C.-M. Chou, M. Suginome, Org. Lett. 2008, 10, 377–380.
- 10H. Noguchi, K. Hojo, M. Suginome, J. Am. Chem. Soc. 2007, 129, 758–759.
- 11L. T. Ball, G. C. Lloyd-Jones, C. A. Russell, J. Am. Chem. Soc. 2014, 136, 254–264.
- 12R. Kumar, A. Linden, C. Nevado, J. Am. Chem. Soc. 2016, 138, 12790–13793.
- 13M. G. Mclaughlin, C. A. McAdam, M. J. Cook, Org. Lett. 2015, 17, 10–13.
- 14C. Reus, N.-W. Liu, M. Bolte, H.-W. Lerner, M. Wagner, J. Org. Chem. 2012, 77, 3518–3523.
- 15A. S. K. Hashmi, D. F. Toste, Modern gold catalyzed synthesis, Wiley, Hoboken, 2012.
10.1002/9783527646869 Google Scholar
- 16G. Zhang, Y. Peng, L. Cui, L. Zhang, Angew. Chem. Int. Ed. 2009, 48, 3112–3115; Angew. Chem. 2009, 121, 3158–3161.
- 17A. D. Melhado, W. E. Brenzovich, A. D. Lackner, F. D. Toste, J. Am. Chem. Soc. 2010, 132, 8885–8887.
- 18T. de Haro, C. Nevado, J. Am. Chem. Soc. 2010, 132, 1512–1513.
- 19H. Peng, Y. Xi, N. Ronaghi, B. Dong, N. G. Akhmedov, X. Shi, J. Am. Chem. Soc. 2014, 136, 13174–13177.
- 20M. N. Hopkinson, A. D. Gee, V. Gouverneur, Chem. Eur. J. 2011, 17, 8248–8262.
- 21L. T. Ball, G. C. LIoyd-Jones, C. A. Russell, Science 2012, 337, 1644–1648.
- 22T. J. A. Corrie, L. T. Ball, C. A. Russell, G. C. LIoyd-Jones, J. Am. Chem. Soc. 2017, 139, 245–254.
- 23A. J. Cresswell, G. C. Lloyd-Jones, Chem. Eur. J. 2016, 22, 12641–12645.
- 24M. Hofer, A. Genoux, R. Kumar, C. Nevado, Angew. Chem. Int. Ed. 2017, 56, 1021–1025; Angew. Chem. 2017, 129, 1041–1045.
- 25Q. Wu, C. Du, Y. Huang, X. Liu, Z. Long, F. Song, J. You, Chem. Sci. 2015, 6, 288–293.
- 26X. C. Cambeiro, N. Ahlsten, I. Larrosa, J. Am. Chem. Soc. 2015, 137, 15636–15639.
- 27M. N. Hopkinson, A. Tlahuext-Aca, F. Glorius, Acc. Chem. Res. 2016, 49, 2261–2272.
- 28B. Sahoo, M. N. Hopkinson, F. Glorius, J. Am. Chem. Soc. 2013, 135, 5505–5508.
- 29A. Tlahuext-Aca, M. N. Hopkinson, C. G. Daniliuc, F. Glorius, Chem. Eur. J. 2016, 22, 11587–11592.
- 30X. Shu, M. Zhang, Y. He, H. Frei, F. D. Toste, J. Am. Chem. Soc. 2014, 136, 5844–5847.
- 31S. Kim, J. Rojas-Martin, F. D. Toste, Chem. Sci. 2016, 7, 85–88.
- 32D. V. Patil, H. Yun, S. Shin, Adv. Synth. Catal. 2015, 357, 2622–2628.
- 33V. Gauchot, A.-L. Lee, Chem. Commun. 2016, 52, 10163–10166.
- 34T. Cornilleau, P. Hermange, E. Fouquet, Chem. Commun. 2016, 52, 10040–10043.
- 35E. O. Asomeza-Solís, J. Rojas-Ocampo, R. A. Toscanoa, S. Porcel, Chem. Commun. 2016, 52, 7295–7298.
- 36A. Zeinddine, L. Estévez, L. S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, Nat. Commun. 2017, 565.
- 37L. Huang, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2016, 55, 4808–4813; Angew. Chem. 2016, 128, 4888–4893.
- 38L. Huang, F. Rominger, M. Rudolph, A. S. K. Hashmi, Chem. Commun. 2016, 52, 6435–6438.
- 39S. Witzel, J. Xie, M. Rudolph, A. S. K. Hashmi, Adv. Synth. Catal. 2017, 359, 1522–1528.
- 40H. Kawai, W. J. Wolf, A. G. DiPasquale, M. S. Winston, F. D. Toste, J. Am. Chem. Soc. 2016, 138, 587–593.
- 41CCDC 1535250 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 42M. Schlosser, Angew. Chem. Int. Ed. 2006, 45, 5432–5446; Angew. Chem. 2006, 118, 5558–5572.
- 43Q. Zhang, Z.-Q. Zhang, Y. Fu, H.-Z. Yu, ACS Catal. 2016, 6, 798–808.
- 44W. E. Brenzovich, J.-F. Brazeau, F. D. Toste, Org. Lett. 2010, 12, 4728–4731.
- 45D. P. Hari, B. König, Angew. Chem. Int. Ed. 2013, 52, 4734–4743; Angew. Chem. 2013, 125, 4832–4842.
- 46S. K. Pawar, M.-C. Yang, M.-D. Su, R.-S. Liu, Angew. Chem. Int. Ed. 2017, 56, 5035–5039; Angew. Chem. 2017, 129, 5117–5121.
- 47R. R. Singh, R.-S. Liu, Chem. Commun. 2017, 53, 4593–4596.