Metal-Free Synthesis of Pharmaceutically Important Biaryls by Photosplicing
Graphical Abstract
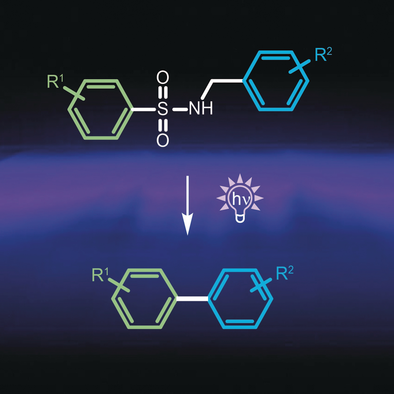
Light on biaryls: A novel metal-free approach to biaryls is reported that involves the tethering and photochemical fusion of phenyl groups through sulfonamides. Using a flow reactor biaryl pharmacophores of numerous therapeutics, antibiotics, antitumor and neuroprotective agents, non-steroidal antiinflammatory drugs, sartans, and cannabinol, were prepared in excellent yields.
Abstract
Many pharmaceuticals feature biaryl motifs that are crucial for their binding to the target. Yet, benchmark methods for selective cross-couplings rely on highly toxic heavy metal catalysts, which are unfavorable in the synthesis of pharmaceuticals. Metal-free coupling reactions, on the other hand, may require harsh conditions and lack selectivity. We report a novel, metal-free cross-coupling reaction that involves the tethering of two phenyl groups by a temporary, traceless sulfonamide linker that directs a photochemical aryl fusion into a single coupling product. The perfect regio- and chemoselectivity of the reaction could be rationalized by a cyclic intermediate, which fragments into the biaryl and volatile side products. Using a flow reactor, we synthesized numerous substituted biaryl building blocks for important therapeutics in high yields, such as antibiotics, antitumor, neuroprotective and cholesterol-lowering agents as well as antiarthritic non-steroidal antiinflammatory drugs (NSAIDs). The new method was successfully employed in a total synthesis of cannabinol, an important analgesic and antiemetic therapeutic. We also report a metal-free synthesis of key building blocks used for the preparation of sartans, antihypertensive agents that rank among the top blockbuster drugs worldwide. This safe and convenient protocol is a valuable alternative for the widely used metal-dependent aryl cross-coupling methods.