Enantioselective Synthesis of the Spirotropanyl Oxindole Scaffold through Bimetallic Relay Catalysis
Dr. Zhi-Jun Jia
Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Technische Universität Dortmund, Fakultät Chemie, Chemische Biologie, Chemische Biologie, Otto-Hahn-Strasse 4a, 44227 Dortmund, Germany
Search for more papers by this authorDr. Gang Shan
Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Search for more papers by this authorDr. Constantin G. Daniliuc
Westfälische Wilhelms-Universität Münster, Organisch-Chemisches Institut, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Dr. Andrey P. Antonchick
Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Technische Universität Dortmund, Fakultät Chemie, Chemische Biologie, Chemische Biologie, Otto-Hahn-Strasse 4a, 44227 Dortmund, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Herbert Waldmann
Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Technische Universität Dortmund, Fakultät Chemie, Chemische Biologie, Chemische Biologie, Otto-Hahn-Strasse 4a, 44227 Dortmund, Germany
Search for more papers by this authorDr. Zhi-Jun Jia
Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Technische Universität Dortmund, Fakultät Chemie, Chemische Biologie, Chemische Biologie, Otto-Hahn-Strasse 4a, 44227 Dortmund, Germany
Search for more papers by this authorDr. Gang Shan
Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Search for more papers by this authorDr. Constantin G. Daniliuc
Westfälische Wilhelms-Universität Münster, Organisch-Chemisches Institut, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Dr. Andrey P. Antonchick
Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Technische Universität Dortmund, Fakultät Chemie, Chemische Biologie, Chemische Biologie, Otto-Hahn-Strasse 4a, 44227 Dortmund, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Herbert Waldmann
Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Technische Universität Dortmund, Fakultät Chemie, Chemische Biologie, Chemische Biologie, Otto-Hahn-Strasse 4a, 44227 Dortmund, Germany
Search for more papers by this authorGraphical Abstract
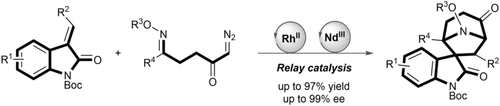
Ring, ring: The first enantioselectively catalyzed synthesis of the spirotropanyl oxindole scaffold, which is characteristic of bioactive alkaloids like alstonisine and chitosenine, was developed. The method involves a bimetallic relay catalysis strategy, with a highly enantioselective 1,3-dipolar cycloaddition as the key step.
Abstract
Spirotropanyl oxindole alkaloids like alstonisine and chitosenine show a wide range of bioactivites. We report the first enantioselective synthesis of the spirotropanyl oxindole scaffold by means of a bimetallic relay catalysis strategy. A new class of E-oximino α-diazo ketones was developed for the intramolecular generation of transient azomethine ylides catalyzed by an achiral RhII complex and a subsequent intermolecular 1,3-dipolar cycloaddition catalyzed by a chiral N,N′-dioxide NdIII Lewis acid complex. The enantioselectively catalyzed transformation has broad scope and yields the desired spirotropanyl oxindole cycloadducts in high yields and with very high enantio- and diastereoselectivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201712882-sup-0001-misc_information.pdf4.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Grynkiewicz, M. Gadzikowska, Pharmacol. Rep. 2008, 60, 439–463;
- 1bD. O'Hagan, Nat. Prod. Rep. 2000, 17, 435–446;
- 1cD. O'Hagan, Nat. Prod. Rep. 1997, 14, 637–651.
- 2
- 2aS. Cheenpracha, T. Ritthiwigrom, S. Laphookhieo, J. Nat. Prod. 2013, 76, 723–726;
- 2bK. Zaima, I. Koga, N. Iwasawa, T. Hosoya, Y. Hirasawa, T. Kaneda, I. S. Ismail, N. H. Lajis, H. Morita, J. Nat. Med. 2013, 67, 9–16.
- 3
- 3aS. Sakai, N. Aimi, K. Yamaguchi, H. Ohhira, K. Hori, J. Haginiwa, Tetrahedron Lett. 1975, 16, 715–718;
10.1016/S0040-4039(00)71965-4 Google Scholar
- 3bN. Aimi, K. Yamaguchi, S. Sakai, J. Haginiwa, A. Kubo, Chem. Pharm. Bull. 1978, 26, 3444–3449;
- 3cS. Sakai, N. Aimi, K. Yamaguchi, E. Yamanaka, J. Haginiwa, J. Chem. Soc. Perkin Trans. 1 1982, 1257–1262.
- 4
- 4aW.-H. Wong, P.-B. Lim, C.-H. Chuah, Phytochemistry 1996, 41, 313–315;
- 4bC. W. Wright, D. Allen, Y. Cai, J. D. Phillipson, I. M. Said, G. C. Kirby, D. C. Warhurst, Phytother. Res. 1992, 6, 121–124;
- 4cJ. V. Silverton, T. Akiyama, J. Chem. Soc. Perkin Trans. 1 1982, 1263–1267;
- 4dS. Sakai, N. Aimi, A. Kubo, M. Kitagawa, M. Hanasawa, K. Katano, K. Yamaguchi, J. Haginiwa, Chem. Pharm. Bull. 1975, 23, 2805–2817;
- 4eS. Sakai, N. Aimi, K. Yamaguchi, E. Yamanaka, J. Haginiwa, Tetrahedron Lett. 1975, 16, 719–722;
10.1016/S0040-4039(00)71966-6 Google Scholar
- 4fJ. Haginiwa, S. Sakai, A. Kubo, K. Takahashi, M. Taguchi, Yakugaku Zasshi 1970, 90, 219–223.
- 5
- 5aO. A. Namjoshi, J. M. Cook, in The Alkaloids: Chemistry and Biology, Vol. 76 (Ed.: ), Elsevier, Amsterdam, 2016, pp. 63–169;
- 5bY. Tsunematsu, N. Ishikawa, D. Wakana, Y. Goda, H. Noguchi, H. Moriya, K. Hotta, K. Watanabe, Nat. Chem. Biol. 2013, 9, 818–825;
- 5cS. P. Hollinshead, D. S. Grubisha, D. W. Bennett, J. M. Cook, Heterocycles 1989, 29, 529–537;
- 5dR. L. Garnick, P. W. Le Quesne, J. Am. Chem. Soc. 1978, 100, 4213–4219.
- 6Selected reviews:
- 6aG. O. Fonseca, J. M. Cook, Org. Chem. Insights 2016, 6, 1–55; For selected examples:
10.4137/OCI.S17958 Google Scholar
- 6bM. R. Stephen, M. T. Rahman, V. V. N. P. B. Tiruveedhula, G. O. Fonseca, J. R. Deschamps, J. M. Cook, Chem. Eur. J. 2017, 23, 15805–15819;
- 6cG. O. Fonseca, Z.-J. Wang, O. A. Namjoshi, J. R. Deschamps, J. M. Cook, Tetrahedron Lett. 2015, 56, 3052–3056;
- 6dJ. Yang, X. Z. Wearing, P. W. Le QUesne, J. R. Deschamps, J. M. Cook, J. Nat. Prod. 2008, 71, 1431–1440;
- 6eX. Z. Wearing, J. M. Cook, Org. Lett. 2002, 4, 4237–4240;
- 6fH. C. Malinakova, L. S. Liebeskind, Org. Lett. 2000, 2, 4083–4086;
- 6gP. Yu, J. M. Cook, Tetrahedron Lett. 1997, 38, 8799–8802;
- 6hA. C. Peterson, J. M. Cook, Tetrahedron Lett. 1994, 35, 2651–2654.
- 7Selected reviews on biology-oriented synthesis:
- 7aL. Laraia, H. Waldmann, Drug Discovery Today Technol. 2017, 23, 75–82;
- 7bH. van Hattum, H. Waldmann, J. Am. Chem. Soc. 2014, 136, 11853–11859;
- 7cS. Wetzel, R. S. Bon, K. Kumar, H. Waldmann, Angew. Chem. Int. Ed. 2011, 50, 10800–10826; Angew. Chem. 2011, 123, 10990–11018;
- 7dR. S. Bon, H. Waldmann, Acc. Chem. Res. 2010, 43, 1103–1114;
- 7eK. Kumar, H. Waldmann, Angew. Chem. Int. Ed. 2009, 48, 3224–3242; Angew. Chem. 2009, 121, 3272–3290.
- 8Selected examples:
- 8aT. Förster, S. Lopéz-Tosco, S. Ziegler, A. P. Antonchick, H. Waldmann, ChemBioChem 2017, 12, 1098–1108;
- 8bH. Xu, C. Golz, C. Strohmann, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2016, 55, 7761–7765; Angew. Chem. 2016, 128, 7892–7896;
- 8cR. Narayan, J. O. Bauer, C. Strohmann, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2013, 52, 12892–12896; Angew. Chem. 2013, 125, 13130–13134;
- 8dA. P. Antonchick, S. López-Tosco, J. Parga, S. Sievers, M. Schürmann, H. Preut, S. Hóing, H. R. Schöler, J. Sterneckert, D. Rauh, H. Waldmann, Chem. Biol. 2013, 20, 500–509;
- 8eA. P. Antonchick, H. Schuster, H. Bruss, M. Schurmann, H. Preut, D. Rauh, H. Waldmann, Tetrahedron 2011, 67, 10195–10202;
- 8fA. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh, H. Waldmann, Nat. Chem. 2010, 2, 735–740.
- 9Selected reviews of N,N′-dioxide ligands and their applications in asymmetric synthesis:
- 9aX. Liu, H. Zheng, Y. Xia, L. Lin, X. Feng, Acc. Chem. Res. 2017, 50, 2621–2631;
- 9bX. Liu, L. Lin, X. Feng, Org. Chem. Front. 2014, 1, 298–302;
- 9cX. Liu, L. Lin, X. Feng, Acc. Chem. Res. 2011, 44, 574–587; selected examples:
- 9dD. Zhang, C. Yin, Y. Zhou, Y. Xu, L. Lin, X. Liu, X. Feng, Chem. Commun. 2017, 53, 7925–7928;
- 9eC. Yin, L. Lin, D. Zhang, J. Feng, X. Liu, X. Feng, J. Org. Chem. 2015, 80, 9691–9699;
- 9fY. Zhou, L. Lin, C. Yin, Z. Wang, X. Liu, X. Feng, Chem. Commun. 2015, 51, 11689–11692;
- 9gX. Lian, S. Guo, G. Wang, L. Lin, X. Liu, X. Feng, J. Org. Chem. 2014, 79, 7703–7710;
- 9hG. Wang, X. Liu, T. Huang, Y. Kuang, L. Lin, X. Feng, Org. Lett. 2013, 15, 76–79; For the seminal reports:
- 9iY. Shen, X. Feng, G. Zhang, Y. Jiang, Synlett 2002, 1353–1355;
- 9jB. Liu, X. Feng, F. Chen, G. Zhang, X. Cui, Y. Jiang, Synlett 2001, 1551–1554.
- 10Seminal reports of azomethine ylides generated by intramolecular RhII carbenoid transfer:
- 10aA. Padwa, D. C. Dean, M. H. Osterhout, L. Precedo, M. A. Semones, J. Org. Chem. 1994, 59, 5347–5357;
- 10bA. Padwa, D. C. Dean, J. Org. Chem. 1990, 55, 405–406.
- 11Selected reviews on the synthetic applications of carbonyl ylides generated by intramolecular RhII carbenoid transfer:
- 11aD. M. Hodgson, A. H. Labande, S. Muthusamy, Org. React. 2013, 80, 133–157;
- 11bA. Padwa, Tetrahedron 2011, 67, 8057–8072;
- 11cA. Padwa, Chem. Soc. Rev. 2009, 38, 3072–3081;
- 11dA. Padwa, Helv. Chim. Acta 2005, 88, 1357–1374.
- 12See the Supporting Information for further details.
- 13
- 13aM. Moran, G. Bernardinelli, P. Müller, Helv. Chim. Acta 1995, 78, 2048–2052;
- 13bK. B. Hansen, N. S. Finney, E. N. Jacobsen, Angew. Chem. Int. Ed. Engl. 1995, 34, 676–678; Angew. Chem. 1995, 107, 750–752.
- 14Selected examples on the combination of Lewis acid catalysis and RhII catalysis:
- 14aH. Suga, Y. Hashimoto, S. Yasumura, R. Takezawa, K. Itoh, A. Kakehi, J. Org. Chem. 2013, 78, 10840–10852;
- 14bX.-Y. Guan, L.-P. Yang, W. Hu, Angew. Chem. Int. Ed. 2010, 49, 2190–2192; Angew. Chem. 2010, 122, 2236–2238;
- 14cH. Suga, S. Higuchi, M. Ohtsuka, D. Ishimoto, T. Arikawa, Y. Hashimoto, S. Misawa, T. Tsuchida, A. Kakehi, T. Baba, Tetrahedron 2010, 66, 3070–3089;
- 14dH. Suga, T. Suzuki, K. Inoue, A. Kakehi, Tetrahedron 2006, 62, 9218–9225;
- 14eH. Suga, K. Inoue, S. Inoue, A. Kakehi, M. Shiro, J. Org. Chem. 2005, 70, 47–56;
- 14fH. Suga, K. Inoue, S. Inoue, A. Kakehi, J. Am. Chem. Soc. 2002, 124, 14836–14837;
- 14gH. Suga, A. Kakehi, S. Ito, K. Inoue, H. Ishida, T. Ibata, Org. Lett. 2000, 2, 3145–3148.
- 15G. Li, T. Liang, L. Wojtas, J. C. Antilla, Angew. Chem. Int. Ed. 2013, 52, 4628–4632; Angew. Chem. 2013, 125, 4726–4730.
- 16A. L. Gerten, M. C. Slade, K. M. Pugh, L. M. Stanley, Org. Biomol. Chem. 2013, 11, 7834–7837.
- 17
- 17aD. N. Zalatan, J. Du Bois, J. Am. Chem. Soc. 2009, 131, 7558–7559;
- 17bJ. Du Bois, Chemtracts: Org. Chem. 2005, 18, 1–13;
- 17cC. G. Espino, K. W. Fiori, M. Kim, J. Du Bois, J. Am. Chem. Soc. 2004, 126, 15378–15379.
- 18The 3 m-endo isomer was found to be instable and could be converted through equilibration to another isomer 3 m-endo′ under ambient temperature for a prolonged reaction time. The same phenomena were observed for other endo cycloadducts. Through NMR analysis of 3 m-endo′ as shown in the Supporting Information, the equilibrating epimerization of spiro quaternary carbon center was postulated through a Mannich-retro-Mannich type process, which is well known for such spirooxindole moieties. Selected examples:
- 18aJ. Xu, L.-D. Shao, D. Li, X. Deng, Y.-C. Liu, Q.-S. Zhao, C. Xia, J. Am. Chem. Soc. 2014, 136, 17962–17965;
- 18bK. Kong, J. A. Enquist, M. E. McCallum, G. M. Smith, T. Matsumaru, E. Menhaji-Klotz, J. L. Wood, J. Am. Chem. Soc. 2013, 135, 10890–10893;
- 18cG. Laus, J. Chem. Soc. Perkin Trans. 2 1998, 315–318.
- 19CCDC 1590369, 1590370 and 1590371 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.