Radical Alkyne peri-Annulation Reactions for the Synthesis of Functionalized Phenalenes, Benzanthrenes, and Olympicene
Nikolay P. Tsvetkov
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorEdgar Gonzalez-Rodriguez
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorAudrey Hughes
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorGabriel dos Passos Gomes
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorFrankie D. White
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorFebin Kuriakose
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorCorresponding Author
Igor V. Alabugin
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorNikolay P. Tsvetkov
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorEdgar Gonzalez-Rodriguez
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorAudrey Hughes
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorGabriel dos Passos Gomes
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorFrankie D. White
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorFebin Kuriakose
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorCorresponding Author
Igor V. Alabugin
Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306-4390 USA
Search for more papers by this authorGraphical Abstract
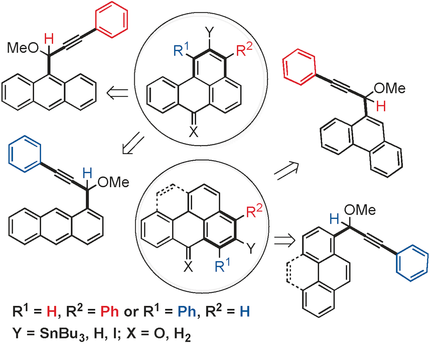
peri useful: Radical cyclization reactions at a peri position were used for the synthesis of polyaromatic compounds. Depending on the choice of reaction conditions and substrate, this flexible approach led to Bu3Sn-substituted phenalene, benzanthrene, and olympicene derivatives (see scheme). Subsequent reactions with electrophiles provided synthetic access to previously inaccessible functionalized polyaromatic compounds.
Abstract
Radical cyclization reactions at a peri position were used for the synthesis of polyaromatic compounds. Depending on the choice of reaction conditions and substrate, this flexible approach led to Bu3Sn-substituted phenalene, benzanthrene, and olympicene derivatives. Subsequent reactions with electrophiles provided synthetic access to previously inaccessible functionalized polyaromatic compounds.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201712783-sup-0001-misc_information.pdf5.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aFor an excellent review of APEX, see: H. Ito, K. Ozaki, K. Itami, Angew. Chem. Int. Ed. 2017, 56, 11144; Angew. Chem. 2017, 129, 11296; for selected recent examples, see:
- 1bW. Matsuoka, H. Ito, K. Itami, Angew. Chem. Int. Ed. 2017, 56, 12224; Angew. Chem. 2017, 129, 12392;
- 1cK. Ozaki, K. Murai, W. Matsuoka, K. Kawasumi, H. Ito, K. Itami, Angew. Chem. Int. Ed. 2017, 56, 1361; Angew. Chem. 2017, 129, 1381.
- 2
- 2aE. Chernick, R. Tykwinski, J. Phys. Org. Chem. 2013, 26, 742;
- 2bI. V. Alabugin, B. Gold, J. Org. Chem. 2013, 78, 7777;
- 2cfor selected non-annulative reactions of alkynes in the synthesis of polyaromatics, see: V. S. Iyer, M. Wehmeier, J. D. Brand, M. A. Keegstra, K. Müllen, Angew. Chem. Int. Ed. Engl. 1997, 36, 1604; Angew. Chem. 1997, 109, 1676;
- 2dX. Feng, W. Pisula, M. Takase, X. Dou, V. Enkelmann, M. Wagner, N. Ding, K. Müllen, Chem. Mater. 2008, 20, 2872.
- 3
- 3aT. J. Hill, R. K. Hughes, L. T. Scott, Tetrahedron 2008, 64, 11360;
- 3bE. H. Fort, P. M. Donovan, L. T. Scott, J. Am. Chem. Soc. 2009, 131, 16006;
- 3c Fragments of Fullerenes and Carbon Nanotubes: Designed Synthesis, Unusual Reactions, and Coordinate Chemistry (Eds.: ), Wiley, New York, 2011;
- 3dfor further creative applications, see: E. P. Jackson, T. J. Sisto, E. R. Darzi, R. Jasti, Tetrahedron 2016, 72, 3754.
- 4
- 4aM. B. Goldfinger, K. B. Crawford, T. M. Swager, J. Org. Chem. 1998, 63, 1676;
- 4bJ. D. Tovar, T. M. Swager, J. Organomet. Chem. 2002, 653, 215.
- 5
- 5aW. Yang, J. H. S. K. Monteiro, A. de Bettencourt-Dias, V. J. Catalano, W. A. Chalifoux, Angew. Chem. Int. Ed. 2016, 55, 10427; Angew. Chem. 2016, 128, 10583;
- 5bW. Yang, J. H. S. K. Monteiro, A. de Bettencourt-Dias, W. A. Chalifoux, Can. J. Chem. 2017, 95, 341.
- 6
- 6aC.-W. Li, C.-I. Wang, H.-Y. Liao, R. Chaudhuri, R.-S. Liu, J. Org. Chem. 2007, 72, 9203;
- 6bR. K. Mohamed, S. Mondal, J. V. Guerrera, T. M. Eaton, T. E. Albrecht-Schmitt, M. Shatruk, I. V. Alabugin, Angew. Chem. Int. Ed. 2016, 55, 12054; Angew. Chem. 2016, 128, 12233;
- 6cR. Chaudhuri, M.-Y. Hsu, C.-W. Li, C.-I. Wang, C.-J. Chen, C. K. Lai, L.-Y. Chen, S.-H. Liu, C.-C. Wu, R.-S. Liu, Org. Lett. 2008, 10, 3053;
- 6dC. Dou, S. Saito, L. Gao, N. Matsumoto, T. Karasawa, H. Zhang, A. Fukazawa, S. Yamaguchi, Org. Lett. 2013, 15, 80.
- 7
- 7aW. Yang, A. Lucotti, M. Tommasini, W. A. Chalifoux, J. Am. Chem. Soc. 2016, 138, 9137;
- 7bW. Yang, W. A. Chalifoux, Synlett 2017, 28, 625.
- 8
- 8aC. Nevado, A. M. Echavarren, Synthesis 2005, 2, 167; for the platinum- and gold-catalyzed hydroarylation of alkynes, see:
- 8bV. Mamane, P. Hannen, A. Fürstner, Chem. Eur. J. 2004, 10, 4556;
- 8cC. Nevado, A. M. Echavarren, Chem. Eur. J. 2005, 11, 3155;
- 8dM. T. Reetz, K. Sommer, Eur. J. Org. Chem. 2003, 3485.
- 9The regioselectivity of ring closure can originate from alternative mechanistic pathways:
- 9aM. Bandini, E. Emer, S. Tommasi, A. Umani-Ronchi, Eur. J. Org. Chem. 2006, 3527;
- 9bN. Chernyak, V. Gevorgyan, Adv. Synth. Catal. 2009, 351, 1101;
- 9cK. Komeyama, R. Igawa, K. Takaki, Chem. Commun. 2010, 46, 1748.
- 10H. Arslan, F. J. Uribe-Romo, B. J. Smith, W. R. Dichtel, Chem. Sci. 2013, 4, 3973.
- 11K. Pati, C. Michas, D. Allenger, I. Piskun, P. S. Coutros, G. dos Passos Gomes, I. V. Alabugin, J. Org. Chem. 2015, 80, 11706.
- 12
- 12aY.-T. Wu, K.-H. Huang, C.-C. Shin, T.-C. Wu, Chem. Eur. J. 2008, 14, 6697;
- 12bS. Mochida, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Lett. 2010, 39, 744;
- 12cM. V. Pham, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 3484; Angew. Chem. 2014, 126, 3552;
- 12dY. Matsuda, S. Naoe, S. Oishi, N. Fujii, H. Ohno, Chem. Eur. J. 2015, 21, 1463.
- 13P. Nun, S. Gaillard, A. Poater, L. Cavallo, S. P. Nolan, Org. Biomol. Chem. 2011, 9, 101.
- 14P. Byers, I. V. Alabugin, J. Am. Chem. Soc. 2012, 134, 9609.
- 15In our earlier designs, the propargylic directing group was “traceless” because it was removed by aromatization of the newly formed ring:
- 15aK. Pati, G. dos Passos Gomes, T. Harris, A. Hughes, H. Phan, T. Banerjee, K. Hanson, I. V. Alabugin, J. Am. Chem. Soc. 2015, 137, 1165;
- 15bK. Pati, G. dos Passos Gomes, I. V. Alabugin, Angew. Chem. Int. Ed. 2016, 55, 11633; Angew. Chem. 2016, 128, 11805;
- 15cfor mechanistic analysis of the C−O scission, see: T. Harris, G. dos Passos Gomes, R. J. Clark, I. V. Alabugin, J. Org. Chem. 2016, 81, 6007.
- 16
- 16aZ. Mou, T. Kubo, M. Kertesz, Chem. Eur. J. 2015, 21, 18230;
- 16bK. Uchida, T. Kubo, J. Synth. Org. Chem. Jpn. 2016, 74, 1069;
- 16cK. Goto, T. Kubo, K. Yamamoto, K. Nakasuji, K. Sato, D. Shiomi, T. Takui, M. Kubota, T. Kobayashi, K. Yakusi, J. Ouyang, J. Am. Chem. Soc. 1999, 121, 1619;
- 16dK. Uchida, Z. Mou, M. Kertesz, T. Kubo, J. Am. Chem. Soc. 2016, 138, 4665.
- 17For the synthesis and electrochemistry of related polyaromatic ketones, see: S. F. Vasilevsky, D. S. Baranov, V. I. Mamatyuk, D. S. Fadeev, Y. V. Gatilov, A. A. Stepanov, N. V. Vasilieva, I. V. Alabugin, J. Org. Chem. 2015, 80, 1618.
- 18
- 18aA. Mistry, B. Moreton, B. Schuler, F. Mohn, G. Meyer, L. Gross, A. Williams, P. Scott, G. Costantini, D. J. Fox, Chem. Eur. J. 2015, 21, 2011;
- 18bA. J. S. Valentine, D. A. Mazziotti, J. Phys. Chem. A 2013, 117, 9746.
- 19Although this value is lower than the experimental BDE of Bu3Sn−H (ca. 78 kcal mol−1), the difference between the calculated C−H and Sn−H BDEs agrees perfectly with the experimentally observed selectivity.
- 20A. Studer, Chem. Eur. J. 2001, 7, 1159.
10.1002/1521-3765(20010316)7:6<1159::AID-CHEM1159>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar