Associative Covalent Relay: An Oxadiazolone Strategy for Rhodium(III)-Catalyzed Synthesis of Primary Pyridinylamines
Xiaolong Yu
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorKehao Chen
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorQi Wang
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorShan Guo
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorShanke Zha
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin Zhu
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorXiaolong Yu
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorKehao Chen
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorQi Wang
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorShan Guo
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorShanke Zha
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin Zhu
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Chemistry for Life Sciences, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorGraphical Abstract
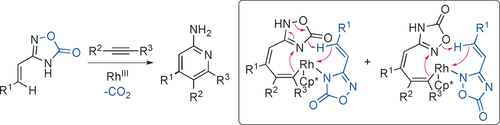
A relay formalism is proposed herein for categorizing the interplay among reactants, target product, and catalytic center in transition-metal catalysis, an important factor dictating the overall reaction viability and efficiency. An intriguing associative covalent relay process in RhIII-catalyzed oxadiazolone-directed alkenyl C−H coupling with alkynes enables efficient access to primary pyridinylamines.
Abstract
A relay formalism is proposed herein for categorizing the interplay among reactants, target product, and catalytic center in transition-metal catalysis, an important factor that can dictate overall catalysis viability and efficiency. In this formalism, transition-metal catalysis can proceed by dissociative relay, associative covalent relay, and associative dative relay modes. An intriguing associative covalent relay process operates in rhodium(III)-catalyzed oxadiazolone-directed alkenyl C−H coupling with alkynes and allows efficient access to primary pyridinylamines. Although the primary pyridinylamine synthesis mechanism is posteriori rationalized, the relay formalism formulated herein can provide an important mechanistic conceptual framework for future catalyst design and reaction development.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201700320-sup-0001-misc_information.pdf6.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aX.-X. Guo, D.-W. Gu, Z. Wu, W. Zhang, Chem. Rev. 2015, 115, 1622–1651;
- 1bL. Yang, H. Huang, Chem. Rev. 2015, 115, 3468–3517;
- 1cZ. Huang, H. N. Lim, F. Mo, M. C. Young, G. Dong, Chem. Soc. Rev. 2015, 44, 7764–7786;
- 1dT. Gensch, M. N. Hopkinson, F. Glorius, J. Wencel-Delord, Chem. Soc. Rev. 2016, 45, 2900–2936;
- 1eM. Moselage, J. Li, L. Ackermann, ACS Catal. 2016, 6, 498–525;
- 1fQ.-Z. Zheng, N. Jiao, Chem. Soc. Rev. 2016, 45, 4590–4627;
- 1gJ. A. Labinger, Chem. Rev. 2017, DOI 10.1021/acs.chemrev.6b00583.
- 2J. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, 2009.
- 3
- 3aB. Liu, Y. Fan, Y. Gao, C. Sun, C. Xu, J. Zhu, J. Am. Chem. Soc. 2013, 135, 468–473;
- 3bB. Liu, C. Song, C. Sun, S. Zhou, J. Zhu, J. Am. Chem. Soc. 2013, 135, 16625–16631;
- 3cA. K. Cook, S. D. Schimler, A. J. Matzger, M. S. Sanford, Science 2016, 351, 1421–1424;
- 3dJ. J. Topczewski, P. J. Cabrera, N. I. Saper, M. S. Sanford, Nature 2016, 531, 220–224;
- 3eJ. H. Kim, S. Greßies, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 5577–5581; Angew. Chem. 2016, 128, 5667–5671;
- 3fN. Y. P. Kumar, A. Bechtoldt, K. Raghuvanshi, L. Ackermann, Angew. Chem. Int. Ed. 2016, 55, 6929–6932; Angew. Chem. 2016, 128, 7043–7046;
- 3gT. Gensch, F. J. R. Klauck, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 11287–11291; Angew. Chem. 2016, 128, 11457–11461;
- 3hP. Wang, G.-C. Li, P. Jain, M. E. Farmer, J. He, P.-X. Shen, J.-Q. Yu, J. Am. Chem. Soc. 2016, 138, 14092–14099.
- 4Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu, Y. Zhang, Org. Chem. Front. 2015, 2, 1107–1295.
- 5
- 5aN. Chatani, A. Kamitani, S. Murai, J. Org. Chem. 2002, 67, 7014–7018;
- 5bD. A. Colby, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2008, 130, 3645–3651;
- 5cS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585–9587;
- 5dD. R. Stuart, P. Alsabeh, M. Kuhn, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 18326–18339;
- 5eT. K. Hyster, T. Rovis, Chem. Commun. 2011, 47, 11846–11848;
- 5fS. Duttwyler, C. Lu, A. L. Rheingold, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2012, 134, 4064–4067;
- 5gK. Sasano, J. Takaya, N. Iwasawa, J. Am. Chem. Soc. 2013, 135, 10954–10957;
- 5hS. Duttwyler, S. Chen, M. K. Takase, K. B. Wiberg, R. G. Bergman, J. A. Ellman, Science 2013, 339, 678–682;
- 5iS. Kujawa, D. Best, D. J. Burns, H. W. Lam, Chem. Eur. J. 2014, 20, 8599–8602;
- 5jA. Seoane, N. Casanova, N. Quiñones, J. L. Mascareñas, M. Gulías, J. Am. Chem. Soc. 2014, 136, 7607–7610.
- 6X. Yu, K. Chen, F. Yang, S. Zha, J. Zhu, Org. Lett. 2016, 18, 5412–5415.
- 7
- 7aC. Chen, K. M. Wilcoxen, C. Q. Huang, Y.-F. Xie, J. R. McCarthy, T. R. Webb, Y.-F. Zhu, J. Saunders, X.-J. Liu, T.-K. Chen, H. Bozigian, D. E. Grigoriadis, J. Med. Chem. 2004, 47, 4787–4798;
- 7bS. Ueda, H. Nagasawa, J. Am. Chem. Soc. 2009, 131, 15080–15080;
- 7cN. Chernyak, V. Gevorgyan, Angew. Chem. Int. Ed. 2010, 49, 2743–2746; Angew. Chem. 2010, 122, 2803–2806;
- 7dJ. Zeng, Y. J. Tan, M. L. Leow, X.-W. Liu, Org. Lett. 2012, 14, 4386–4389;
- 7eS. Santra, A. K. Bagdi, A. Majee, A. Hajraa, Adv. Synth. Catal. 2013, 355, 1065–1070;
- 7fI. I. Roslan, Q.-X. Lim, A. Han, G.-K. Chuah, S. Jaenicke, Eur. J. Org. Chem. 2015, 2351–2355;
- 7gO. P. S. Patel, D. Anand, R. K. Maurya, P. P. Yadav, Green Chem. 2015, 17, 3728–3732.
- 8
- 8aK. Parthasarathy, M. Jeganmohan, C.-H. Cheng, Org. Lett. 2008, 10, 325–329;
- 8bK. Parthasarathy, C.-H. Cheng, Synthesis 2009, 8, 1400–1402;
- 8cD.-S. Kim, J.-W. Park, C.-H. Jun, Chem. Commun. 2012, 48, 11334–11336;
- 8dR. M. Martin, R. G. Bergman, J. A. Ellman, J. Org. Chem. 2012, 77, 2501–2507;
- 8eJ. M. Neely, T. Rovis, J. Am. Chem. Soc. 2013, 135, 66–69;
- 8fJ. M. Neely, T. Rovis, J. Am. Chem. Soc. 2014, 136, 2735–2738;
- 8gD. Majee, S. Biswas, S. M. Mobin, S. Samanta, J. Org. Chem. 2016, 81, 4378–4385;
- 8hH. Jiang, J. Yang, X. Tang, J. Li, W. Wu, J. Org. Chem. 2015, 80, 8763–8771;
- 8iT. M. M. Maiden, S. Swanson, P. A. Procopiou, J. P. A. Harrity, Org. Lett. 2016, 18, 3434–3437.
- 9D. Kumar, S. R. Vemula, G. R. Cook, ACS Catal. 2016, 6, 3531–3536.
- 10CCDC 1534641 (3 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 11
- 11aG. Liu, Y. Shen, Z. Zhou, X. Lu, Angew. Chem. Int. Ed. 2013, 52, 6033–6037; Angew. Chem. 2013, 125, 6149–6153;
- 11bH. Zhang, K. Wang, B. Wang, H. Yi, F. Hu, C. Li, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2014, 53, 13234–13238; Angew. Chem. 2014, 126, 13450–13454.
- 12CCDC 1534640 (1 b-Rh-WM) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 13K. T. J. Loones, B. U. W. Maes, R. A. Dommisse, G. L. F. Lemière, Chem. Commun. 2004, 2466–2467.