Controlling Proton Delivery through Catalyst Structural Dynamics
Graphical Abstract
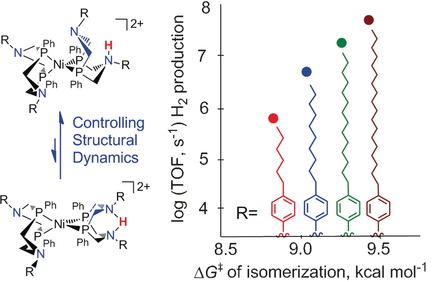
Slower dynamics, faster catalysis: Dynamic processes that are key to catalysis can be controlled through remote substituents in the outer coordination sphere, in a manner similar to the role of the protein architecture in enzymes. This approach was used to increase the H2 production rates of nickel catalysts by three orders of magnitude with a minimal increase in overpotential.
Abstract
The fastest synthetic molecular catalysts for H2 production and oxidation emulate components of the active site of hydrogenases. The critical role of controlled structural dynamics is recognized for many enzymes, including hydrogenases, but is largely neglected in designing synthetic catalysts. Our results demonstrate the impact of controlling structural dynamics on H2 production rates for [Ni(PPh2NC6H4R2)2]2+ catalysts (R=n-hexyl, n-decyl, n-tetradecyl, n-octadecyl, phenyl, or cyclohexyl). The turnover frequencies correlate inversely with the rates of chair–boat ring inversion of the ligand, since this dynamic process governs protonation at either catalytically productive or non-productive sites. These results demonstrate that the dynamic processes involved in proton delivery can be controlled through modification of the outer coordination sphere, in a manner similar to the role of the protein architecture in many enzymes. As a design parameter, controlling structural dynamics can increase H2 production rates by three orders of magnitude with a minimal increase in overpotential.