A Stable Crystalline Triarylphosphine Oxide Radical Anion
Graphical Abstract
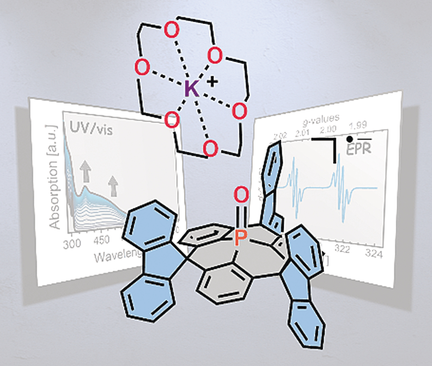
The spin within: A carefully designed triarylphosphine oxide scaffold with sterically demanding spirofluorenyl moieties undergoes chemical one-electron reduction at its phosphoryl moiety. The unique stability of the formed radical anion enables the isolation and X-ray crystallographic characterization of this hitherto elusive species.
Abstract
Triarylphosphine oxides (Ar3P=O) are being intensely studied as electron-accepting (n-type) materials. Despite the widespread application of these compounds as electron conductors, experimental data regarding the structural and electronic properties of their negatively charged states remain scarce owing to their propensity for follow-up chemistry. Herein, a carefully designed triarylphosphine oxide scaffold is disclosed that comprises sterically demanding spirofluorenyl moieties to shield the central phosphoryl (P=O) moiety. This compound undergoes chemical one-electron reduction to afford an exceptionally stable radical anion, which was isolated and characterized by X-ray crystallography for the very first time. The experimental data, corroborated by computational studies, shall allow for the construction of phosphine oxide materials with enhanced stability.