Low-Temperature Transformation of Methane to Methanol on Pd1O4 Single Sites Anchored on the Internal Surface of Microporous Silicate
Graphical Abstract
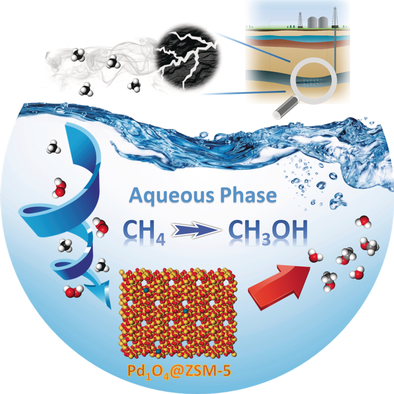
Single site Pd1O4 anchored in microspores of zeolite with 2.0 w % CuO is active for transforming of CH4 to CH3OH in aqueous solution in the temperature range of 50–95 °C. Selectivity for production of CH3OH in this temperature range was found to be 78 %-86 % at 50–95 °C, offering a clear improvement over harsh alternative conditions.
Abstract
Direct conversion of methane to chemical feedstocks such as methanol under mild conditions is a challenging but ideal solution for utilization of methane. Pd1O4 single-sites anchored on the internal surface of micropores of a microporous silicate exhibit high selectivity and activity in transforming CH4 to CH3OH at 50–95 °C in aqueous phase through partial oxidation of CH4 with H2O2. The selectivity for methanol production remains at 86.4 %, while the activity for methanol production at 95 °C is about 2.78 molecules per Pd1O4 site per second when 2.0 wt % CuO is used as a co-catalyst with the Pd1O4@ZSM-5. Thermodynamic calculations suggest that the reaction toward methanol production is highly favorable compared to formation of a byproduct, methyl peroxide.