Photooxygenation of Furylalkylamines: Easy Access to Pyrrolizidine and Indolizidine Scaffolds
Dr. Dimitris Kalaitzakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorMyron Triantafyllakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorManolis Sofiadis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorDr. Dimitris Noutsias
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorCorresponding Author
Prof. Dr. Georgios Vassilikogiannakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorDr. Dimitris Kalaitzakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorMyron Triantafyllakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorManolis Sofiadis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorDr. Dimitris Noutsias
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorCorresponding Author
Prof. Dr. Georgios Vassilikogiannakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003 Iraklion, Crete, Greece
Search for more papers by this authorGraphical Abstract
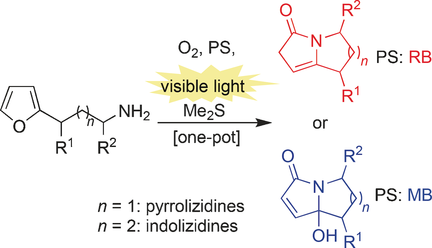
Singlet oxygen is able to transform unprotected primary furylalkylamines into the important pyrrolizidine and indolizidine scaffolds. The outcome of the one-pot sequences can be readily tailored to need by varying the choice of sensitizer. The synthetic utility of this method was demonstrated in the rapid synthesis of five natural products. PS: Photosensitizer. RB: rose Bengal. MB: Methylene blue.
Abstract
A highly adaptable method targeting the ubiquitous and very important pyrrolizidine and indolizidine scaffolds is presented. The general synthetic utility of the method is underscored by its application to the rapid and easy synthesis of five natural products starting from readily accessible alkylfuran precursors. These unprotected primary furylalkylamines are subjected to photooxygenation conditions, which initiate a complex cascade reaction sequence concluding with the production of high value motifs. This sequence can be tailored to need by varying the choice of both photosensitizer and base additive.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201600988-sup-0001-misc_information.pdf5.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. M. Garraffo, T. F. Spande, J. W. Daly, A. Baldessari, E. G. Gros, J. Nat. Prod. 1993, 56, 357–373;
- 1bH. M. Garraffo, J. Caceres, J. W. Daly, T. F. Spande, N. R. Andriamaharavo, M. Andriantsiferana, J. Nat. Prod. 1993, 56, 1016–1038.
- 2D. S. Seigler in Plant Secondary Metabolism, Spinger Science + Business Media, New York, 1998, pp. 546–567.
10.1007/978-1-4615-4913-0_30 Google Scholar
- 3R. A. Saporito, M. A. Donnelly, R. A. Norton, H. M. Garraffo, T. F. Spande, J. W. Daly, Proc. Natl. Acad. Sci. USA 2007, 104, 8885–8890.
- 4T. H. Jones, H. L. Voegtle, H. M. Miras, R. G. Weatherford, T. F. Spande, H. M. Garraffo, J. W. Daly, D. W. Davidson, R. R. Snelling, J. Nat. Prod. 2007, 70, 160–168.
- 5J. W. Daly, T. F. Spande, H. M. Garraffo, J. Nat. Prod. 2005, 68, 1556–1575.
- 6J. W. Daly, Proc. Natl. Acad. Sci. USA 1995, 92, 9–13.
- 7
- 7aJ. W. Daly, C. W. Myers, N. Whittaker, Toxicon 1987, 25, 1023–1095;
- 7bR. S. Aronstam, J. W. Daly, T. F. Spande, T. K. Narayanan, E. X. Albuquerque, Neurochem. Res. 1986, 11, 1227–1240;
- 7cA. El-Shazly, M. Wink, Diversity 2014, 6, 188–282;
- 7dP. L. Katavic, D. A. Venables, T. Rali, A. R. Carroll, J. Nat. Prod. 2007, 70, 872–875;
- 7eB. Singh, P. M. Sahu, S. C. Jain, S. Singh, Pharm. Biol. 2002, 40, 581–586;
- 7fT. Hartmann, L. Witte in The Alkaloids: Chemical and Biological Perspectives, Vol. 9 (Ed.: ), Pergamon, Oxford, 1995, pp. 155–233.
- 8R. Nakai, H. Ogawa, A. Asai, K. Ando, T. Agatsuma, S. Matsumiya, S. Akinaga, Y. Yamashita, T. Mizukami, J. Antibiot. 2000, 53, 294–296.
- 9B. Tasso, F. Novelli, F. Sparatore, F. Fasoli, C. Gotti, J. Nat. Prod. 2013, 76, 727–731.
- 10S. H. Goh, W. Chen, A. R. M. Ali, Tetrahedron Lett. 1984, 25, 3483–3484.
- 11T. Agatsuma, T. Akama, S. Nara, S. Matsumiya, R. Nakai, H. Ogawa, S. Otaki, S. Ikeda, Y. Saitoh, Y. Kanda, Org. Lett. 2002, 4, 4387–4390.
- 12H. Takahata, M. Kubota, K. Ihara, N. Okamoto, T. Momose, N. Azer, A. T. Eldefrawi, M. E. Eldefrawi, Tetrahedron: Asymmetry 1998, 9, 3289–3301.
- 13J. W. Daly, E. McNeal, F. Gusovksy, F. Ito, L. E. Overman, J. Med. Chem. 1988, 31, 477–480.
- 14B. K. Cassels, I. Bermúdez, F. Dajas, J. A. Abin-Carriquiry, S. Wonnacott, Drug Discovery Today 2005, 10, 1657–1665.
- 15
- 15aJ. P. Michael, Nat. Prod. Rep. 2008, 25, 139–165;
- 15bJ. Robertson, K. Stevens, Nat. Prod. Rep. 2014, 31, 1721–1788;
- 15cA. Brandi, F. Cardona, S. Cicchi, F. M. Cordero, A. Goti, Chem. Eur. J. 2009, 15, 7808–7821;
- 15dC. Bhat, S. G. Tilve, RSC Adv. 2014, 4, 5405–5452.
- 16
- 16aS. Nicolai, C. Piemontesi, J. Waser, Angew. Chem. Int. Ed. 2011, 50, 4680–4683; Angew. Chem. 2011, 123, 4776–4779;
- 16bG. Lapointe, K. Schenk, P. Renaud, Chem. Eur. J. 2011, 17, 3207–3212;
- 16cT. Jiang, T. Livinghouse, H. M. Lovick, Chem. Commun. 2011, 47, 12861–12863;
- 16dF. Abels, C. Lindemann, E. Koch, C. Schneider, Org. Lett. 2012, 14, 5972–5975;
- 16eS. V. Pronin, M. G. Tabor, D. J. Jansen, R. A. Shenvi, J. Am. Chem. Soc. 2012, 134, 2012–2015;
- 16fN. Ortega, D.-T. D. Tang, S. Urban, D. Zhao, F. Glorius, Angew. Chem. Int. Ed. 2013, 52, 9500–9503; Angew. Chem. 2013, 125, 9678–9681;
- 16gD. Koley, Y. Krishna, K. Srinivas, A. A. Khan, R. Kant, Angew. Chem. Int. Ed. 2014, 53, 13196–13200; Angew. Chem. 2014, 126, 13412–13416;
- 16hY. Kang, M. T. Richers, C. H. Sawicki, D. Seidel, Chem. Commun. 2015, 51, 10648–10651.
- 17
- 17aD. Kalaitzakis, T. Montagnon, I. Alexopoulou, G. Vassilikogiannakis, Angew. Chem. Int. Ed. 2012, 51, 8868–8871; Angew. Chem. 2012, 124, 8998–9001;
- 17bD. Kalaitzakis, T. Montagnon, E. Antonatou, N. Bardají, G. Vassilikogiannakis, Chem. Eur. J. 2013, 19, 10119–10123;
- 17cD. Kalaitzakis, T. Montagnon, E. Antonatou, G. Vassilikogiannakis, Org. Lett. 2013, 15, 3714–3717;
- 17dD. Kalaitzakis, E. Antonatou, G. Vassilikogiannakis, Chem. Commun. 2014, 50, 400–402;
- 17eD. Kalaitzakis, A. Kouridaki, D. Noutsias, T. Montagnon, G. Vassilikogiannakis, Angew. Chem. Int. Ed. 2015, 54, 6283–6287; Angew. Chem. 2015, 127, 6381–6385.
- 18T. Montagnon, D. Kalaitzakis, M. Triantafyllakis, M. Stratakis, G. Vassilikogiannakis, Chem. Commun. 2014, 50, 15480–15498.
- 19
- 19aE. Baciocchi, T. Del Giacco, O. Lanzalunga, A. Lapi, J. Org. Chem. 2007, 72, 9582–9589;
- 19bG. Jiang, J. Chen, J.-S. Huang, C.-M. Che, Org. Lett. 2009, 11, 4568–4571;
- 19cA. Okada, H. Yuasa, A. Fujiya, N. Tada, T. Miura, A. Itoh, Synlett 2015, 1705–1709.
- 20
- 20aI. V. Trushkov, M. G. Uchuskin, A. V. Butin, Eur. J. Org. Chem. 2015, 2999–3016;
- 20bS. Naud, S. J. Macnaughton, B. S. Dyson, D. J. Woollaston, J. W. P. Dallimore, J. Robertson, Org. Biomol. Chem. 2012, 10, 3506–3518.
- 21M. F. Brackeen, D. J. Cowan, J. A. Stafford, F. J. Schoenen, J. M. Veal, P. L. Domanico, D. Rose, A. B. Strickland, M. Verghese, P. L. Feldman, J. Med. Chem. 1995, 38, 4848–4854.
- 22M. J. Martín-López, F. Bermejo-González, Tetrahedron Lett. 1994, 35, 8843–8846.
- 23M. O. Rasmussen, P. Delair, A. E. Greene, J. Org. Chem. 2001, 66, 5438–5443.
- 24J. W. Daly in Progress in the Chemistry of Organic Natural Products, Vol. 41 (Eds.: ), Springer, Vienna, 1982, pp. 205–340.
- 25F. L. Warren, M. E. von Klemperer, J. Chem. Soc. 1958, 4574–4575.
- 26Y.-C. Tsai, M.-L. Yu, M. El-Shazly, L. Beerhues, Y.-B. Cheng, L.-C. Chen, T.-L. Hwang, H.-F. Chen, Y.-M. Chung, M.-F. Hou, Y.-C. Wu, F.-R. Chang, J. Nat. Prod. 2015, 78, 2346–2354.