Highly Enantioselective Rhodium-Catalyzed Addition of Arylboroxines to Simple Aryl Ketones: Efficient Synthesis of Escitalopram
Linwei Huang
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
Search for more papers by this authorJinbin Zhu
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
Search for more papers by this authorGuangjun Jiao
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorDr. Zheng Wang
Innovation Center China, AstraZeneca Global R&D, China
Search for more papers by this authorProf. Dr. Xingxin Yu
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei-Ping Deng
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenjun Tang
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorLinwei Huang
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
Search for more papers by this authorJinbin Zhu
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
Search for more papers by this authorGuangjun Jiao
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorDr. Zheng Wang
Innovation Center China, AstraZeneca Global R&D, China
Search for more papers by this authorProf. Dr. Xingxin Yu
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei-Ping Deng
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenjun Tang
Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Rd, Shanghai, 200237 China
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
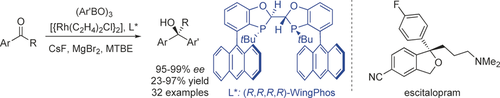
Wing it: The title reaction has been achieved for the first time with a Rh/(R,R,R,R)-WingPhos catalyst, providing a range of chiral diaryl alkyl carbinols with excellent ee values and yields. The method was applied for a concise synthesis of the antidepressant drug escitalopram. MTBE=tert-butyl methyl ether.
Abstract
Highly enantioselective additions of arylboroxines to simple aryl ketones have been achieved for the first time with a Rh/(R,R,R,R)-WingPhos catalyst, thus providing a range of chiral diaryl alkyl carbinols with excellent ee values and yields. (R,R,R,R)-WingPhos has been proven to be crucial for the high reactivity and enantioselectivity. The method has enabled a new, concise, and enantioselective synthesis of the antidepressant drug escitalopram.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201600979-sup-0001-misc_information.pdf4.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1a Comprehensive Asymmetric Catalysis (Eds.: ), Springer, Berlin, 1999;
- 1bL. Pu, H.-B. Yu, Chem. Rev. 2001, 101, 757;
- 1cK. Fagnou, K. M. Lautens, Chem. Rev. 2003, 103, 169;
- 1dS. E. Denmark, J. Fu, Chem. Rev. 2003, 103, 2763;
- 1eC. Garcia, V. S. Martin, Curr. Org. Chem. 2006, 10, 1849;
- 1fO. Riant, J. Hannedouche, Org. Biomol. Chem. 2007, 5, 873;
- 1gP. Tian, H.-Q. Dong, G.-Q. Lin, ACS Catal. 2012, 2, 95;
- 1hD. Ameen, T. J. Snape, MedChemComm 2013, 4, 893.
- 2
- 2aS. Dhillon, L. J. Scott, G. L. Plosker, CNS Drugs 2006, 20, 763;
- 2bA. M. Fournier, R. A. Brown, W. Farnaby, H. Miyatake-Ondozabal, J. Clayden, Org. Lett. 2010, 12, 2222;
- 2cL. A. Nathan, J. Appl. Ther. 1962, 4, 830;
- 2dT. A. Yap, M. I. Walton, K. M. Grimshaw, R. H. te Poele, P. D. Eve, M. R. Valanie, A. K. de Haven Brandon, V. Martins, A. Zetterlund, S. P. Heaton, K. Heinzmann, P. S. Jones, R. E. Feltell, M. Reule, S. J. Woodhead, T. G. Davies, J. F. Lyons, F. I. Raynaud, S. A. Eccles, P. Workman, N. E. Thompson, M. D. Garrett, Clin. Cancer Res. 2012, 18, 3912;
- 2eM. Chang, T. H. Kim, H.-D. Kim, Tetrahedron: Asymmetry 2008, 19, 1504;
- 2fR. Wallace, A. L. Porte, R. Hodges, J. Chem. Soc. 1963, 1445.
- 3For other methods such as asymmetric additions of various carbon nucleophiles to aromatic ketones, asymmetric dihydroxylation of styrenes, and tertiary boronic acids, see:
- 3aM. Shibasaki, M. Kanai, Chem. Rev. 2008, 108, 2853;
- 3bM. Hatano, K. Ishihara, Synthesis 2008, 1647;
- 3cM. C. Noe, M. A. Letavic, S. L. Snow, Org. React. 2005, 66, 109;
- 3dJ. L. Stymiest, V. Bagutski, R. M. French, V. K. Aggarwal, Nature 2008, 456, 778;
- 3eD. Leonori, V. K. Aggarwal, Acc. Chem. Res. 2014, 47, 3174.
- 4For enantioselective organozinc addition to ketones, see:
- 4aD. J. Ramón, M. Yus, Angew. Chem. Int. Ed. 2004, 43, 284; Angew. Chem. 2004, 116, 286;
- 4bJ. M. Betancort, C. García, P. J. Walsh, Synlett 2004, 749;
- 4cP. I. Dosa, G. C. Fu, J. Am. Chem. Soc. 1998, 120, 445;
- 4dD. J. Ramón, M. Yus, Tetrahedron Lett. 1998, 39, 1239;
- 4eO. Prieto, D. J. Ramón, M. Yus, Tetrahedron: Asymmetry 2003, 14, 1955;
- 4fH. Li, C. García, P. J. Walsh, Proc. Natl. Acad. Sci. USA 2004, 101, 5425; For enantioselective organoaluminum addition to ketones, see:
- 4gC.-A. Chen, K.-H. Wu, H.-M. Gau, Angew. Chem. Int. Ed. 2007, 46, 5373; Angew. Chem. 2007, 119, 5469.
- 5For amino- or iminoalcohol-catalyzed asymmetric arylation in combination with an arylboronic acid (or ester) and diethylzinc, see:
- 5aC. Bolm, J. Rudolph, J. Am. Chem. Soc. 2002, 124, 14850;
- 5bJ. Rudolph, F. Schmidt, C. Bolm, Adv. Synth. Catal. 2004, 346, 867;
- 5cJ.-X. Ji, J. Wu, T. T.-L. Au-Yeung, C.-W. Yip, R. K. Haynes, A. S. C. Chan, J. Org. Chem. 2005, 70, 1093;
- 5dA. L. Braga, D. S. Lüdtke, ; F. Vargas, M. W. Paixão, Chem. Commun. 2005, 2512; F. Vargas, M. W. Paixão, Chem. Commun. 2005, 2512;
- 5eG. Lu, F. Y. Kwong, J.-W. Ruan, Y.-M. Li, A. S. C. Chan, Chem. Eur. J. 2006, 12, 4115;
- 5fN. A. Magnus, P. B. Anzeveno, D. S. Coffey, D. A. Hay, M. E. Laurila, J. M. Schkeryantz, B. W. Shaw, M. A. Staszak, Org. Process Res. Dev. 2007, 11, 560;
- 5gJ. Ruan, G. Lu, L. Xu, Y.-M. Li, A. S. C. Chan, Adv. Synth. Catal. 2008, 350, 76.
- 6For rhodium catalysts, see:
- 6aM. Sakai, M. Ueda, N. Miyaura, Angew. Chem. Int. Ed. 1998, 37, 3279;
10.1002/(SICI)1521-3773(19981217)37:23<3279::AID-ANIE3279>3.0.CO;2-M CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 3475;10.1002/(SICI)1521-3757(19981204)110:23<3475::AID-ANGE3475>3.0.CO;2-W Web of Science® Google Scholar
- 6bT. Focken, J. Rudolph, C. Bolm, Synthesis 2005, 429;
- 6cK. Suzuki, S. Ishii, K. Kondo, T. Aoyama, Synlett 2006, 648;
- 6dK. Suzuki, K. Kondo, T. Aoyama, Synthesis 2006, 1360;
- 6eT. Arao, K. Suzuki, K. Kondo, T. Aoyama, Synthesis 2006, 3809;
- 6fH.-F. Duan, J.-H. Xie, W.-J. Shi, Q. Zhang, Q.-L. Zhou, Org. Lett. 2006, 8, 1479;
- 6gR. B. C. Jagt, P. Y. Toullec, E. P. Schudde, J. G. de Vries, B. L. Feringa, A. J. Minnaard, J. Comb. Chem. 2007, 9, 407;
- 6hS. Morikawa, K. Michigami, H. Amii, Org. Lett. 2010, 12, 2520;
- 6iR. B. C. Jagt, P. Y. Toullec, J. G. de Vries, B. L. Feringa, A. J. Minnaard, Org. Biomol. Chem. 2006, 4, 773;
- 6jT. Nishimura, H. Kumamoto, M. Nagaosa, T. Hayashi, Chem. Commun. 2009, 5713;
- 6kC.-H. Xing, Y.-X. Liao, P. He, Q.-S. Hu, Chem. Commun. 2010, 46, 3010; For ruthenium catalysts, see:
- 6lY. Yamamoto, K. Kurihara, N. Miyaura, Angew. Chem. Int. Ed. 2009, 48, 4414; Angew. Chem. 2009, 121, 4478;
- 6mK. Li, N. Hu, R. Luo, W. Yuan, W. Tang, J. Org. Chem. 2013, 78, 6350; For copper catalysts, see:
- 6nD. Tomita, M. Kanai, M. Shibasaki, Chem. Chem. Asian J. 2006, 1, 161; For nickel catalysts, see:
- 6oT. Arao, K. Kondo, T. Aoyama, Tetrahedron Lett. 2007, 48, 4115;
- 6pK. Yamamoto, K. Tsurumi, F. Sakurai, K. Kondo, T. Aoyama, Synthesis 2008, 3585;
- 6qF. Sakurai, K. Kondo, T. Aoyama, Chem. Pharm. Bull. 2009, 57, 511;
- 6rF. Sakurai, K. Kondo, T. Aoyama, Tetrahedron Lett. 2009, 50, 6001.
- 7For additions to α-keto esters, see:
- 7aH.-F. Duan, J.-H. Xie, X.-C. Qiao, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2008, 47, 4351; Angew. Chem. 2008, 120, 4423;
- 7bY. Yamamoto, T. Shirai, M. Watanabe, K. Kurihara, N. Miyaura, Molecules 2011, 16, 5020;
- 7cF. Cai, X. Pu, X. Qi, V. Lynch, A. Radha, J. M. Ready, J. Am. Chem. Soc. 2011, 133, 18066;
- 7dT.-S. Zhu, S.-S. Jin, M.-H. Xu, Angew. Chem. Int. Ed. 2012, 51, 780; Angew. Chem. 2012, 124, 804; For additions to isatins, see:
- 7eR. Shintani, M. Inoue, T. Hayashi, Angew. Chem. Int. Ed. 2006, 45, 3353; Angew. Chem. 2006, 118, 3431;
- 7fJ. Gui, G. Chen, P. Cao, J. Liao, Tetrahedron: Asymmetry 2012, 23, 554;
- 7gP. Y. Toullec, R. B. C. Jagt, J. G. de Vries, B. L. Feringa, A. J. Minnaard, Org. Lett. 2006, 8, 2715;
- 7hH. Lai, Z. Huang, Q. Wu, Y. Qin, J. Org. Chem. 2009, 74, 283;
- 7iZ. Liu, P. Gu, M. Shi, P. McDowell, G. Li, Org. Lett. 2011, 13, 2314. For additions to trifluoromethyl ketones, see:
- 7jS. L. X. Martina, R. B. C. Jagt, J. G. de Vries, B. L. Feringa, A. J. Minnaard, Chem. Commun. 2006, 4093;
- 7kV. R. Jumde, S. Facchetti, A. Iuliano, Tetrahedron: Asymmetry 2010, 21, 2775;
- 7lR. Luo, K. Li, Y. Hu, W. Tang, Adv. Synth. Catal. 2013, 355, 1297.
- 8
- 8aG. Liu, X. Lu, J. Am. Chem. Soc. 2006, 128, 16504;
- 8bG. Liu, X. Lu, Tetrahedron 2008, 64, 7324;
- 8cD. W. Low, G. Pattison, M. D. Wieczysty, G. H. Churchill, H. W. Lam, Org. Lett. 2012, 14, 2548.
- 9
- 9aJ. Bouffard, K. Itami, Org. Lett. 2009, 11, 4410;
- 9bT. Korenaga, A. Ko, K. Uotani, Y. Tanaka, T. Sakai, Angew. Chem. Int. Ed. 2011, 50, 10703; Angew. Chem. 2011, 123, 10891;
- 9cY.-X. Liao, C.-H. Xing, Q.-S. Hu, Org. Lett. 2012, 14, 1544.
- 10
- 10aW. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029;
- 10bJ.-H. Xie, S.-F. Zhu, Q.-L. Zhou, Chem. Rev. 2011, 111, 1713;
- 10cW. Tang, X. Zhang, Angew. Chem. Int. Ed. 2002, 41, 1612;
10.1002/1521-3773(20020503)41:9<1612::AID-ANIE1612>3.0.CO;2-H CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1682.
- 11G. Liu, X. Liu, Z. Cai, G. Jiao, G. Xu, W. Tang, Angew. Chem. Int. Ed. 2013, 52, 4235; Angew. Chem. 2013, 125, 4329.
- 12
- 12aW. Tang, B. Qu, A. G. Capacci, S. Rodriguez, X. Wei, N. Haddad, B. Narayanan, S. Ma, N. Grinberg, N. K. Yee, D. Krishnamurthy, C. H. Senanayake, Org. Lett. 2010, 12, 176;
- 12bD. R. Fandrick, K. R. Fandrick, J. T. Reeves, Z. Tan, W. Tang, A. G. Capacci, S. Rodriguez, J. J. Song, H. Lee, N. K. Yee, C. H. Senanayake, J. Am. Chem. Soc. 2010, 132, 7600.
- 13DFT calculations were performed with a Gaussian 03 package and the geometries were optimized with UB3LYP and a mixed basis set of 3-21G for all atoms.
- 14For resolution of racemates, see:
- 14aK. P. Boegesoe, J. Perregaard, EP347066A1, 1989;
- 14bK. P. Boegesoe, EP171943A1, 1986;
- 14cG. Cotticelli, R. Salvetti, C. Bertoni, WO2006136521A1, 2006;
- 14dC. R. Elati, N. Kolla, H. Vurimidi, V. T. Mathad, Org. Process Res. Dev. 2007, 11, 289;
- 14eR. M. Pulla, R. T. Sambasiva, C. N. Venkaiah, WO2006025071A1, 2006.
- 15For reported asymmetric syntheses, see:
- 15aM. Albert, H. Sturm, A. Berger, P. Kremminger, WO2007082771A1, 2007;
- 15bB. M. Partridge, S. P. Thomas, V. K. Aggarwal, Tetrahedron 2011, 67, 10082.