Carborane Substituents Promote Direct Electrophilic Insertion over Reduction–Metalation Reactions
Graphical Abstract
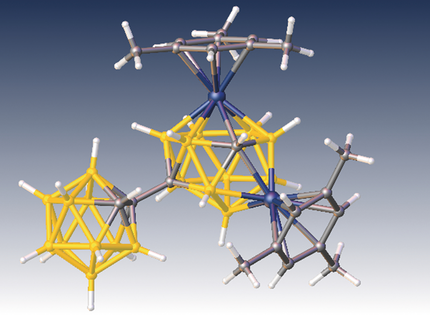
Take two: Two-electron reduction of 1,1′-bis(o-carborane) and subsequent metalation with {Ru(mes)}2+ results in a 4,1,8-RuC2B10 species. A further two-electron reduction allows a direct electrophilic insertion reaction promoted by the carborane substituent for the addition of a second metal fragment and the formation of 1,13,2,9-Ru2C2B10. A mechanism is proposed to rationalize the observed fluxionality of the products.
Abstract
Two-electron reduction of 1,1′-bis(o-carborane) followed by reaction with [Ru(η-mes)Cl2]2 affords [8-(1′-1′,2′-closo-C2B10H11)-4-(η-mes)-4,1,8-closo-RuC2B10H11]. Subsequent two-electron reduction of this species and treatment with [Ru(η-arene)Cl2]2 results in the 14-vertex/12-vertex species [1-(η-mes)-9-(1′-1′,2′-closo-C2B10H11)-13-(η-arene)-1,13,2,9-closo-Ru2C2B10H11] by direct electrophilic insertion, promoted by the carborane substituent in the 13-vertex/12-vertex precursor. When arene=mesitylene (mes), the diruthenium species is fluxional in solution at room temperature in a process that makes the metal–ligand fragments equivalent. A unique mechanism for this fluxionality is proposed and is shown to be fully consistent with the observed fluxionality or nonfluxionality of a series of previously reported 14-vertex dicobaltacarboranes.