Characterization of the Intermediate in and Identification of the Repair Mechanism of (6-4) Photolesions by Photolyases
Corresponding Author
Dr. Shirin Faraji
Interdisciplinary Center for Scientific Computing, Ruprecht-Karls University Heidelberg, Im Neuenheimer Feld 205A, 69120 Heidelberg, Germany
Search for more papers by this authorProf. Dr. Dongping Zhong
Department of Physics, Department of Chemistry and Biochemistry, and Programs of Biophysics, Chemical Physics and Biochemistry, The Ohio State University, Columbus, OH, 43210 USA
Search for more papers by this authorProf. Dr. Andreas Dreuw
Interdisciplinary Center for Scientific Computing, Ruprecht-Karls University Heidelberg, Im Neuenheimer Feld 205A, 69120 Heidelberg, Germany
Search for more papers by this authorCorresponding Author
Dr. Shirin Faraji
Interdisciplinary Center for Scientific Computing, Ruprecht-Karls University Heidelberg, Im Neuenheimer Feld 205A, 69120 Heidelberg, Germany
Search for more papers by this authorProf. Dr. Dongping Zhong
Department of Physics, Department of Chemistry and Biochemistry, and Programs of Biophysics, Chemical Physics and Biochemistry, The Ohio State University, Columbus, OH, 43210 USA
Search for more papers by this authorProf. Dr. Andreas Dreuw
Interdisciplinary Center for Scientific Computing, Ruprecht-Karls University Heidelberg, Im Neuenheimer Feld 205A, 69120 Heidelberg, Germany
Search for more papers by this authorGraphical Abstract
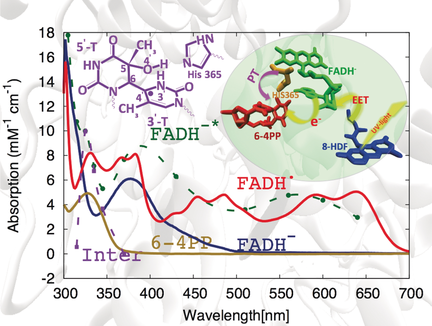
QM/MM simulations were conducted to assign a specific molecular structure to the intermediate formed in the electron-induced repair of (6-4) photolesions (purple) detected previously in ultrafast transient absorption spectroscopy. The repair mechanism involves proton transfer from the protonated His365 to the N3′ nitrogen of the lesion along an oxetane-like transition state.
Abstract
Quantum mechanics/molecular mechanics calculations are employed to assign previously recorded experimental spectroscopic signatures of the intermediates occurring during the photo-induced repair of (6-4) photolesions by photolyases to specific molecular structures. Based on this close comparison of experiment and theory it is demonstrated that the acting repair mechanism involves proton transfer from the protonated His365 to the N3′ nitrogen of the lesion, which proceeds simultaneously with intramolecular OH transfer along an oxetane-like transition state.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201511950-sup-0001-misc_information.pdf1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. L. Carrier, R. D. Snyder, J. D. Regan, The Science of Photomedicine, Plenum, New York, 1982.
10.1007/978-1-4684-8312-3_4 Google Scholar
- 2J.-S. Taylor, Pure Appl. Chem. 1995, 67, 183–190.
- 3R. P. Sinha, D.-P. Hader, Photochem. Photobiol. Sci. 2002, 1, 225–236.
- 4J. T. Reardon, A. Sancar, Genes Dev. 2003, 17, 2539–2551.
- 5S. Weber, Biochim. Biophys. Acta Bioenerg. 2005, 1707, 1–23.
- 6L. O. Essen, T. Klar, Cell. Mol. Life Sci. 2006, 63, 1266–1277.
- 7A. Sancar, J. Biol. Chem. 2008, 283, 32153–32157.
- 8D. B. Sanders, O. Wiest, J. Am. Chem. Soc. 2010, 121, 5127–5134.
- 9S. Faraji, A. Dreuw, Annu. Rev. Phys. Chem. 2014, 65, 275–292.
- 10T. Todo, H. Takemori, H. Ryo, M. Ihara, T. Matsunaga, O. Nikaido, K. Sato, T. Nomura, Nature 1993, 361, 371–374.
- 11C. S. Rupert, S. H. Goodgal, R. M. Herriott, J. Gen. Physiol. 1958, 41, 451–471.
- 12I. Baccarelli, I. Bald, F. A. Gianturco, E. Illenberger, J. Kopyra, Phys. Rep. 2011, 508, 1–44.
- 13B. J. Vande Berg, G. B. Sancar, J. Biol. Chem. 1998, 273, 20276–20284.
- 14J. Antony, D. M. Medvedev, A. Stuchebrukhov, J. Am. Chem. Soc. 2000, 122, 1057–1065.
- 15C. Tan, L. Guo, Y. Ai, J. Li, L. Wang, A. Sancar, Y. Luo, D. Zhong, J. Phys. Chem. A 2014, 118, 10522–10530.
- 16P. H. P. Harbach, M. Schneider, S. Faraji, A. Dreuw, J. Phys. Chem. Lett. 2013, 4, 943–949.
- 17Z. Liu, C. Tan, X. Guo, J. Li, L. Wang, A. Sancar, D. Zhong, Proc. Natl. Acad. Sci. USA 2013, 110, 12972–12977.
- 18C. Tan, Z. Liu, J. Li, X. Guo, L. Wang, A. Sancar, D. Zhong, Nat. Commun. 2015, 6, 7302.
- 19Z. Liu, X. Guo, C. Tan, J. Li, Y. T. Kao, L. Wang, A. Sancar, D. Zhong, J. Am. Chem. Soc. 2012, 134, 8104–8114.
- 20T. Okamura, A. Sancar, P. Heelis, T. Begley, Y. Hirata, N. Mataga, J. Am. Chem. Soc. 1991, 113, 3143–3145.
- 21A. Mees, T. Klar, P. Gnau, U. Hennecke, A. P. M. Eker, T. Carell, Science 2004, 306, 1789–1793.
- 22A. F. Glas, S. Schneider, M. J. Maul, U. Hennecke, T. Carell, Chem. Eur. J. 2009, 15, 10387–10396.
- 23Y. T. Kao, C. Sexena, L. Wang, A. Sancar, D. Zhong, Proc. Natl. Acad. Sci. USA 2005, 102, 16128–16132.
- 24M. J. Maul, T. R. M. Barends, A. F. Glas, M. J. Cryle, T. Domratcheva, S. Schneider, I. Schlichting, T. Carell, Angew. Chem. Int. Ed. 2008, 47, 10076–10080; Angew. Chem. 2008, 120, 10230–10234.
- 25J. Li, Z. Liu, C. Tan, X. Guo, L. Wang, A. Sancar, D. Zhong, Nature 2010, 466, 887–890.
- 26Z. Liu, C. Tan, X. Guo, Y. Kao, J. Li, L. Wang, A. Sancar, D. Zhong, Proc. Natl. Acad. Sci. USA 2011, 108, 14831–14836.
- 27Y. T. Kao, C. Sexena, L. Wang, A. Sancar, D. Zhong, J. Am. Chem. Soc. 2012, 134, 1501–1503.
- 28J. Yamamoto, K. Hitomi, R. Hayashi, E. D. Getzoff, S. Iwai, Biochemistry 2009, 48, 9306–9312.
- 29O. A. Borg, L. A. Eriksson, B. Durbeej, J. Phys. Chem. A 2007, 111, 2351–2361.
- 30X. Zhao, J. Liu, D. S. Hsu, S. Zhao, J. S. Taylor, A. Sancar, J. Biol. Chem. 1997, 272, 32580–32590.
- 31K. Hitomi, H. Nakamura, S. Kim, T. Mizukoshi, T. Ishikawa, S. Iwai, T. Todo, J. Biol. Chem. 2001, 276, 10103–10109.
- 32M. G. Friedel, M. K. Cichon, T. Carell, Org. Biomol. Chem. 2005, 3, 1937–1941.
- 33S. Asgatay, C. Petermann, D. Harakat, D. Guillaume, J. S. Taylor, C. Pascale, J. Am. Chem. Soc. 2008, 130, 12618–12619.
- 34J. Yamamoto, R. Martin, S. Iwai, P. Plaza, K. Brettel, Angew. Chem. Int. Ed. 2013, 52, 7432–7436; Angew. Chem. 2013, 125, 7580–7584.
- 35E. Schleicher, K. Hitomi, C. W. M. Kay, E. D. Getzoff, T. Takeshi, S. Weber, J. Biol. Chem. 2007, 282, 4738–4747.
- 36F. Masson, T. Laino, U. Rothlisberger, J. Hutter, ChemPhysChem 2009, 10, 400–410.
- 37F. Masson, T. Laino, I. Tavernelli, U. Rothlisberger, J. Hutter, J. Am. Chem. Soc. 2008, 130, 3443–3450.
- 38C. B. Harrison, L. L. ONeil, O. Wiest, J. Phys. Chem. A 2005, 109, 7001–7012.
- 39X. Zheng, J. Garcia, A. Stuchebrukhov, J. Phys. Chem. B 2008, 112, 8724–8729.
- 40K. Sadeghian, M. Bocola, T. Merz, M. Schütz, J. Am. Chem. Soc. 2010, 132, 16285–16295.
- 41T. Domratcheva, I. Schlichting, J. Am. Chem. Soc. 2009, 131, 17793–17799.
- 42P. H. P. Harbach, J. Borowka, M.-V. Bohnwagner, A. Dreuw, J. Phys. Chem. Lett. 2010, 1, 2556–2560.
- 43S. Faraji, A. Dreuw, J. Phys. Chem. Lett. 2012, 3, 227–230.
- 44A. Dreuw, S. Faraji, Phys. Chem. Chem. Phys. 2013, 15, 19969–19975.
- 45S. Faraji, L. Wirz, A. Dreuw, ChemPhysChem 2013, 14, 2817–2824.
- 46S. Faraji, G. Groenhof, A. Dreuw, J. Phys. Chem. B 2013, 117, 10071–10079.
- 47T. Domratcheva, J. Am. Chem. Soc. 2011, 133, 18172–18182.
- 48K. Condic-Jurkic, S. Ana-Sunĉana, H. Zipse, D. M. Smith, J. Chem. Theory Comput. 2012, 8, 1078–1091.
- 49Y. M. Rhee, M. Head-Gordon, J. Phys. Chem. A 2007, 111, 5314–5326.
- 50A. Dreuw, M. Wormit, WIREs Comput. Mol. Sci. 2015, 5, 82–95.
- 51O. Christiansen, H. Koch, J. Jørgensen, Chem. Phys. Lett. 1995, 243, 409–418.
- 52D. Zhong, Annu. Rev. Phys. Chem. 2014, 66, 691–715.
- 53Z. Liu, L. Wang, D. Zhong, ChemPhysChem 2015, 17, 11933–11949.