Catalytic and Enantioselective Synthesis of Chiral Multisubstituted Tribenzothiepins by Intermolecular Cycloadditions
Yu-ki Tahara
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorRiku Matsubara
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorAkihito Mitake
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorTatsuki Sato
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorDr. Kyalo Stephen Kanyiva
International Center for Science and Engineering Programs (ICSEP), Waseda University, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Takanori Shibata
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
JST, ACT-C, Kawaguchi, Saitama, 332-0012 Japan
Search for more papers by this authorYu-ki Tahara
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorRiku Matsubara
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorAkihito Mitake
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorTatsuki Sato
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorDr. Kyalo Stephen Kanyiva
International Center for Science and Engineering Programs (ICSEP), Waseda University, Shinjuku, Tokyo, 169-8555 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Takanori Shibata
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555 Japan
JST, ACT-C, Kawaguchi, Saitama, 332-0012 Japan
Search for more papers by this authorGraphical Abstract
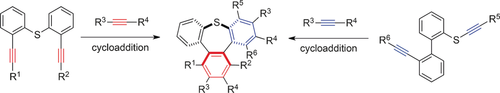
End of the tether: The first catalytic and highly enantioselective synthesis of tribenzothiepin derivatives was achieved. Two types of intermolecular cycloadditions, either using diphenyl-sulfide-tethered diynes or 2-phenyl sulfanylbenzene tethered diynes with a monoalkyne, led to chiral multisubstituted tribenzothiepins in good to excellent ee values under mild reaction conditions. The present protocol was used to prepare a chiral tribenzoselenepin.
Abstract
The first catalytic and highly enantioselective synthesis of tribenzothiepin derivatives was achieved. Two types of intermolecular cycloadditions using either diphenyl-sulfide-tethered diynes or 2-phenyl sulfanylbenzene-tethered diynes with a monoalkyne successfully gave chiral multisubstituted tribenzothiepins in good to excellent ee values under mild conditions. The inversion energy of this saddle-shaped molecule was calculated by measurement of the racemization rate of chiral tribenzothiepins using the Eyring kinetic equation under heating conditions. The present protocol could also be used to prepare a chiral tribenzoselenepin.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201511876-sup-0001-misc_information.pdf7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Yuan, S. Saito, C. Camacho, S. Irle, I. Hisaki, S. Yamaguchi, J. Am. Chem. Soc. 2013, 135, 8842–8845.
- 2H. Huang, T. Stewart, M. Gutmann, T. Ohhara, N. Niimura, Y. X. Li, J. F. Wen, R. Bau, H. N. C. Wong, J. Org. Chem. 2009, 74, 359–369.
- 3
- 3aA. Rajca, A. Safronov, S. Rajca, C. R. Ross, J. J. Stezowski, J. Am. Chem. Soc. 1996, 118, 7272–7279;
- 3bA. Rajca, A. Safronov, S. Rajca, J. Wongsriratanakul, J. Am. Chem. Soc. 2000, 122, 3351–3357;
- 3cA. Rajca, H. Wang, P. Bolshov, S. Rajca, Tetrahedron 2001, 57, 3725–3735;
- 3dT. B. Freedman, X. Cao, A. Rajca, H. Wang, L. A. Nafie, J. Phys. Chem. A 2003, 107, 7692–7696;
- 3eJ. F. Wen, W. Hong, K. Yuan, T. C. W. Mak, H. N. C. Wong, J. Org. Chem. 2003, 68, 8918–8931;
- 3fH. Y. Peng, C. K. Lam, T. C. W. Mak, Z. Cai, W. T. Ma, Y. X. Li, H. N. C. Wong, J. Am. Chem. Soc. 2005, 127, 9603–9611;
- 3gA. H. Wu, C. K. Hau, H. N. C. Wong, Adv. Synth. Catal. 2007, 349, 601–608;
- 3hH. Huang, C. K. Hau, C. C. M. Law, H. N. C. Wong, Org. Biomol. Chem. 2009, 7, 1249–1257;
- 3iT. Shibata, T. Chiba, H. Hirashima, Y. Ueno, K. Endo, Angew. Chem. Int. Ed. 2009, 48, 8066–8069; Angew. Chem. 2009, 121, 8210–8213;
- 3jA. Rajca, S. Rajca, Angew. Chem. Int. Ed. 2010, 49, 672–674; Angew. Chem. 2010, 122, 683–685;
- 3kT. Shibata, T. Uchiyama, H. Hirashima, K. Endo, Pure Appl. Chem. 2011, 83, 597–605;
- 3lT. Shibata, M. Fujimoto, H. Hirashima, T. Chiba, K. Endo, Synthesis 2012, 3269–3284.
- 4K. Yamamoto, S. Yamazaki, Y. Kohashi, I. Murata, Tetrahedron Lett. 1982, 23, 3195–3198.
- 5K. Nishino, M. Takagi, T. Kawata, J. Am. Chem. Soc. 1991, 113, 5059–5060.
- 6
- 6aM. Widhalm, K. Mereiter, Bull. Chem. Soc. Jpn. 2003, 76, 1233–1244;
- 6bL. Dobelmann, A. H. Parham, A. Busing, H. Buchholz, B. Konig, RSC Adv. 2014, 4, 60473–60477.
- 7
- 7aT. Kimura, Y. Ishikawa, N. Furukawa, Heterocycles 1993, 35, 53–56;
- 7bT. Kimura, Y. Ishikawa, K. Ueki, Y. Horie, N. Furukawa, J. Org. Chem. 1994, 59, 7117–7124;
- 7cW. D. Guerra, R. A. Rossi, A. B. Pierini, S. M. Barolo, J. Org. Chem. 2015, 80, 928–941.
- 8
- 8aM. Hori, T. Kataoka, H. Shimizu, M. Okitsu, Chem. Pharm. Bull. 1981, 29, 1244–1251;
- 8bM. Hori, T. Kataoka, H. Shimizu, M. Okitsu, Heterocycles 1981, 15, 1061–1066.
- 9S. Kumar, H. Ila, H. Junjappa, Tetrahedron 2007, 63, 10067–10076.
- 10E. Dimitrijevic, M. Cusimano, M. S. Taylor, Org. Biomol. Chem. 2014, 12, 1391–1394.
- 11W. Tochtermann, C. Franke, Angew. Chem. Int. Ed. Engl. 1967, 6, 370; Angew. Chem. 1967, 79, 319.
- 12
- 12aM. Lautens, W. Klute, W. Tam, Chem. Rev. 1996, 96, 49–92;
- 12bS. Saito, Y. Yamamoto, Chem. Rev. 2000, 100, 2901–2915;
- 12cT. Shibata, K. Tsuchikama, Org. Biomol. Chem. 2008, 6, 1317–1323;
- 12dK. Tanaka, Chem. Asian J. 2009, 4, 508–518;
- 12eY. Shibata, K. Tanaka, Synthesis 2012, 323–350;
- 12f Transition-Metal-Mediated Aromatic Ring Construction, Part I: [2+2+2] and Related Cycloaddition Reactions (Ed.: ), Wiley, Hoboken, 2013.
- 13
- 13aT. Shibata, T. Fujimoto, K. Yokota, K. Takagi, J. Am. Chem. Soc. 2004, 126, 8382–8383;
- 13bT. Shibata, K. Tsuchikama, Chem. Commun. 2005, 6017–6019;
- 13cT. Shibata, Y. Arai, K. Takami, K. Tsuchikama, T. Fujimoto, S. Takebayashi, K. Takagi, Adv. Synth. Catal. 2006, 348, 2475–2483;
- 13dT. Shibata, S. Yoshida, Y. Arai, M. Otsuka, K. Endo, Tetrahedron 2008, 64, 821–830;
- 13eT. Shibata, T. Uchiyama, Y. Yoshinami, S. Takayasu, K. Tsuchikama, K. Endo, Chem. Commun. 2012, 48, 1311–1313;
- 13fT. Shibata, Y. Kamimura, Tetrahedron: Asymmetry 2015, 26, 41–45;
- 13gY. Tahara, R. Matsubara, T. Shibata, Heterocycles 2015, 90, 1094–1110.
- 14
- 14aT. Shibata, K. Tsuchikama, M. Otsuka, Tetrahedron: Asymmetry 2006, 17, 614–619;
- 14bT. Shibata, T. Uchiyama, K. Endo, Org. Lett. 2009, 11, 3906–3908;
- 14cT. Shibata, T. Chiba, H. Hirashima, Y. Ueno, K. Endo, Heteroat. Chem. 2011, 22, 363–370;
- 14dT. Shibata, M. Miyoshi, T. Uchiyama, K. Endo, N. Miura, K. Monde, Tetrahedron 2012, 68, 2679–2686.
- 15
- 15aT. Shibata, M. Fujimoto, T. Otani, Tetrahedron 2014, 70, 8453–8461;
- 15bY. Tahara, M. Gake, R. Matsubara, T. Shibata, Org. Lett. 2014, 16, 5980–5983.
- 16Optimization of the catalyst for the intermolecular cycloaddition using 1 a with DMAD is listed in the Supporting Information.
- 17Optimization of the catalyst for the intermolecular cycloaddition using 4 with DMAD is listed in the Supporting Information.
- 18Q. Tan, S. Higashibayashi, S. Karanjit, H. Sakurai, Nat. Commun. 2012, 3, 891.
- 19Examination for the racemization of tribenzothiepin derivatives is shown in the Supporting Information.
- 20CCDC CCDC 1441339 (3 ga) and 1441340 (5 da) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.