Membrane-permeable Triphosphate Prodrugs of Nucleoside Analogues
Graphical Abstract
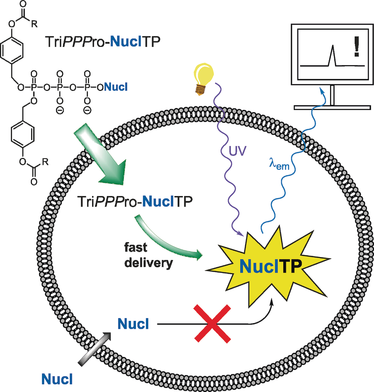
NTP drugs go pro: The TriPPPro-approach was used to synthesize a series of nucleoside triphosphate analogues. The TriPPPro-compounds displayed anti-HIV activity and cellular uptake. In some cases, even inactive parent nucleosides were converted into powerful antiviral compounds. These results could thus aid the development of future nucleoside prodrugs.
Abstract
The metabolic conversion of nucleoside analogues into their triphosphates often proceeds insufficiently. Rate-limitations can be at the mono-, but also at the di- and triphosphorylation steps. We developed a nucleoside triphosphate (NTP) delivery system (TriPPPro-approach). In this approach, NTPs are masked by two bioreversible units at the γ-phosphate. Using a procedure involving H-phosphonate chemistry, a series of derivatives bearing approved, as well as potentially antivirally active, nucleoside analogues was synthesized. The enzyme-triggered delivery of NTPs was demonstrated by pig liver esterase, in human T-lymphocyte cell extracts and by a polymerase chain reaction using a prodrug of thymidine triphosphate. The TriPPPro-compounds of some HIV-inactive nucleoside analogues showed marked anti-HIV activity. For cellular uptake studies, a fluorescent TriPPPro-compound was prepared that delivered the triphosphorylated metabolite to intact CEM cells.