Block Copolymerization of Lactide and an Epoxide Facilitated by a Redox Switchable Iron-Based Catalyst
Graphical Abstract
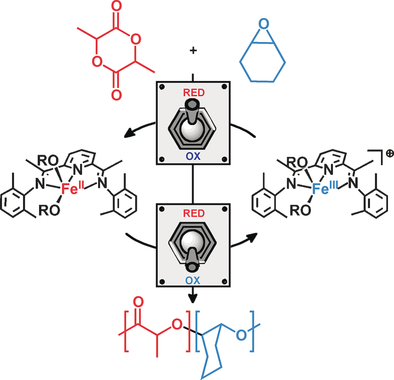
The redox-controlled block copolymerization of cyclohexene oxide and lactide capitalizes on the ability for a bis(imino)pyridine iron bisalkoxide complex to polymerize lactide in the iron(II) oxidation state and epoxide in the iron(III) state, but not vice versa. Diblock copolymers were synthesized with both monomers present starting with either the iron(II) or iron(III) catalyst and using an in situ redox switch.
Abstract
A cationic iron(III) complex was active for the polymerization of various epoxides, whereas the analogous neutral iron(II) complex was inactive. Cyclohexene oxide polymerization could be “switched off” upon in situ reduction of the iron(III) catalyst and “switched on” upon in situ oxidation, which is orthogonal to what was observed previously for lactide polymerization. Conducting copolymerization reactions in the presence of both monomers resulted in block copolymers whose identity can be controlled by the oxidation state of the catalyst: selective lactide polymerization was observed in the iron(II) oxidation state and selective epoxide polymerization was observed in the iron(III) oxidation state. Evidence for the formation of block copolymers was obtained from solubility differences, GPC, and DOSY-NMR studies.