Mechanical Reversibility of Strain-Promoted Azide–Alkyne Cycloaddition Reactions
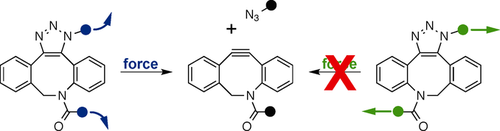
Graphical Abstract
Broken by you: Mechanical cycloreversion of triazoles depends on the direction of the applied force (shearing vs. unzipping) acting on the triazole unit. Computational screening of a number of triazoles formed from strained alkynes shows that cycloreversion only occurs when the force acts in the unzipping geometry and identifies azadibenzylcyclooctyne (DIBAC) as a promising strained alkyne for mechanically reversible triazole structures.
Abstract
Mechanophores, that is, molecules that show a defined response to force, are crucial building blocks of mechanoresponsive materials. The possibility of mechanically induced cycloreversion for a series of triazoles formed via strain-promoted azide–alkyne cycloaddition reactions was investigated by density functional theory calculations, and these triazoles were compared to the 1,4- and 1,5-regioisomers formed in the reaction of an azide with a terminal alkyne. We show that cycloreversion is in principal possible and that the pulling geometry is the most important parameter that determines the probability of cycloreversion. We further compared triazole stability to the mechanical stability of polymers that are frequently used as force transducers in mechanochemical experiments and identified DIBAC (azadibenzylcyclooctyne) as a promising mechanophore for future applications.