Unraveling Inter- and Intrachain Electronics in Polythiophene Assemblies Mediated by Coordination Nanospaces
Michael W. A. MacLean
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, K7L 3N6 (Canada)
Search for more papers by this authorTakashi Kitao
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)
Search for more papers by this authorDr. Takeo Suga
Department of Applied Chemistry, Waseda University, 3-4-1 Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorProf. Dr. Motohiro Mizuno
Department of Chemistry, Graduate School of Natural Science & Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192 (Japan)
Search for more papers by this authorProf. Dr. Shu Seki
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Takashi Uemura
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)
CREST Japan Science and Technology Agency (JST), 4-1-8 Honcho, Kawaguchi, Saitama 332-0012 (Japan)
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)Search for more papers by this authorCorresponding Author
Prof. Susumu Kitagawa
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)
Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501 (Japan)
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)Search for more papers by this authorMichael W. A. MacLean
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, K7L 3N6 (Canada)
Search for more papers by this authorTakashi Kitao
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)
Search for more papers by this authorDr. Takeo Suga
Department of Applied Chemistry, Waseda University, 3-4-1 Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorProf. Dr. Motohiro Mizuno
Department of Chemistry, Graduate School of Natural Science & Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192 (Japan)
Search for more papers by this authorProf. Dr. Shu Seki
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Takashi Uemura
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)
CREST Japan Science and Technology Agency (JST), 4-1-8 Honcho, Kawaguchi, Saitama 332-0012 (Japan)
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)Search for more papers by this authorCorresponding Author
Prof. Susumu Kitagawa
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)
Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501 (Japan)
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan)Search for more papers by this authorGraphical Abstract
Abstract
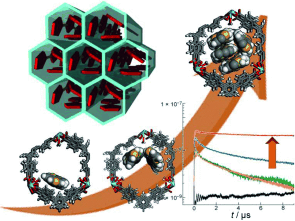
Strong interchain interactions render unsubstituted polythiophene un-fusible, non-melting, and insoluble. Therefore, control of the packing structure, which has a profound effect on the optical and electronic properties of the polymer, has never been achieved. Unsubstituted polythiophene was prepared in the one-dimensional channels of [La(1,3,5-benzenetrisbenzoate)]n, where polymer chains form unprecedented assembly structures mediated by the host framework. It is noteworthy that the emission and carrier transport properties were drastically changed by varying the number of chains within a particular assembly. The response of the composite to additional guests is also examined as a method to use the composites as low-concentration sensors. Our findings show that the encapsulation of polymer chains in host materials is a facile method for understanding the intrinsic properties of conjugated polymers, along with controlling and enhancing their functions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201510084_sm_miscellaneous_information.pdf852.3 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Janata, M. Josowicz, Nat. Mater. 2003, 2, 19–24;
- 1bA. J. Heeger, Chem. Soc. Rev. 2010, 39, 2354–2371.
- 2
- 2aJ. Roncali, Chem. Rev. 1992, 92, 711–738;
- 2bT. Yamamoto, A. Morita, Y. Miyazaki, T. Maruyama, H. Wakayama, Z. H. Zhou, Y. Nakamura, T. Kanbara, S. Sasaki, K. Kubota, Macromolecules 1992, 25, 1214–1223.
- 3
- 3aA. Mishra, C.-Q. Ma, P. Bäuerle, Chem. Rev. 2009, 109, 1141–1276;
- 3bS. Nejati, T. E. Minford, Y. Y. Smolin, K. K. S. Lau, ACS Nano 2014, 8, 5413–5422;
- 3cB. A. G. Hammer, M. A. Reyes-Martinez, F. A. Bokel, F. Liu, T. P. Russell, R. C. Hayward, A. L. Briseno, T. Emrick, ACS Appl. Mater. Interfaces 2014, 6, 7705–7711;
- 3dC. Li, G. Shi, ACS Appl. Mater. Interfaces 2013, 5, 4503–4510;
- 3eV. C. Gonçalves, D. T. Balogh, Sens. Actuators B 2012, 162, 307–312.
- 4
- 4aP. Sozzani, S. Bracco, A. Comotti, P. Valsesia, R. Simonutti, O. Terasaki, Y. Sakamoto, Nat. Mater. 2006, 5, 545–551;
- 4bG. Distefano, A. Comotti, S. Bracco, M. Beretta, P. Sozzani, Angew. Chem. Int. Ed. 2012, 51, 9258–9262; Angew. Chem. 2012, 124, 9392–9396;
- 4cP. Sozzani, A. Comotti, S. Bracco, R. Simonutti, Angew. Chem. Int. Ed. 2004, 43, 2792–2797; Angew. Chem. 2004, 116, 2852–2857;
- 4dT.-Q. Nguyen, J. Wu, V. Doan, B. J. Schwartz, S. H. Tolbert, Science 2000, 288, 652–656;
- 4eA. Comotti, S. Bracco, M. Mauri, S. Mottadelli, T. Ben, S. Qiu, P. Sozzani, Angew. Chem. Int. Ed. 2012, 51, 10136–10140; Angew. Chem. 2012, 124, 10283–10287;
- 4fC.-G. Wu, T. Bein, Science 1994, 264, 1757–1759;
- 4gS. Esnouf, F. Beuneu, P. Enzel, T. Bein, Phys. Rev. B 1997, 56, 12899–12904;
- 4hF. Cucinotta, F. Carniato, G. Paul, S. Bracco, C. Bisio, S. Caldarelli, L. Marchese, Chem. Mater. 2011, 23, 2803–2809;
- 4iJ. Gierschner, L. Lüer, D. Oelkrug, E. Musluoğlu, B. Behnisch, M. Hanack, Adv. Mater. 2000, 12, 757–761;
- 4jD. J. Cardin, Adv. Mater. 2002, 14, 553–563;
- 4kE. Aharon, M. Kalina, G. L. Frey, J. Am. Chem. Soc. 2006, 128, 15968–15969;
- 4lE. Aharon, A. Albo, M. Kalina, G. L. Frey, Adv. Funct. Mater. 2006, 16, 980–986;
- 4mY. Honmou, S. Hirata, H. Komiyama, J. Hiyoshi, S. Kawauchi, T. Iyoda, M. Vacha, Nat. Commun. 2014, 5, 4666;
- 4nM. S. Cho, H. J. Choi, W. S. Ahn, Langmuir 2004, 20, 202–207.
- 5
- 5aM. van den Boogaard, G. Bonnet, P. van’t Hof, Y. Wang, C. Brochon, P. van Hutten, A. Lapp, G. Hadziioannou, Chem. Mater. 2004, 16, 4383–4385;
- 5bP. Enzel, T. Bein, J. Chem. Soc. Chem. Commun. 1989, 1326–1327.
- 6
- 6aO. M. Yaghi, M. O’Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi, J. Kim, Nature 2003, 423, 705–714;
- 6bG. Férey, Chem. Soc. Rev. 2008, 37, 191–214;
- 6cS. Kitagawa, R. Kitaura, S.-i. Noro, Angew. Chem. Int. Ed. 2004, 43, 2334–2375; Angew. Chem. 2004, 116, 2388–2430;
- 6dP. Ramaswamy, N. E. Wong, G. K. H. Shimizu, Chem. Soc. Rev. 2014, 43, 5913–5932.
- 7
- 7aT. Uemura, K. Kitagawa, S. Horike, T. Kawamura, S. Kitagawa, Chem. Commun. 2005, 5968–5970;
- 7bT. Uemura, N. Yanai, S. Watanabe, H. Tanaka, R. Numaguchi, M. T. Miyahara, Y. Ohta, M. Nagaoka, S. Kitagawa, Nat. Commun. 2010, 1, 83;
- 7cT. Uemura, N. Uchida, A. Asano, A. Saeki, S. Seki, M. Tsujimoto, S. Isoda, S. Kitagawa, J. Am. Chem. Soc. 2012, 134, 8360–8363;
- 7dG. Distefano, H. Suzuki, M. Tsujimoto, S. Isoda, S. Bracco, A. Comotti, P. Sozzani, T. Uemura, S. Kitagawa, Nat. Chem. 2013, 5, 335–341.
- 8
- 8aR. M. Metzger, Chem. Rev. 2003, 103, 3803–3834;
- 8bG. Reecht, F. Scheurer, V. Speisser, Y. J. Dappe, F. Mathevet, G. Schull, Phys. Rev. Lett. 2014, 112, 047403.
- 9
- 9aT. Devic, C. Serre, N. Audebrand, J. Marrot, G. Férey, J. Am. Chem. Soc. 2005, 127, 12788–12789;
- 9bJ. Duan, M. Higuchi, S. Horike, M. L. Foo, K. P. Rao, Y. Inubushi, T. Fukushima, S. Kitagawa, Adv. Funct. Mater. 2013, 23, 3525–3530.
- 10This was confirmed through XRPD data (Supporting Information, Figure S3) owing to the lack of peaks corresponding to TTh, indicating that all of the TTh must be present within the pores.
- 11
- 11aK. Kaneko, S. Hayashi, S. Ura, K. Yoshino, J. Phys. Soc. Jpn. 1985, 54, 1146–1153;
- 11bD. Ofer, R. M. Crooks, M. S. Wrighton, J. Am. Chem. Soc. 1990, 112, 7869–7879;
- 11cJ. Chen, J. Shu, S. Schobloch, A. Kroeger, R. Graf, R. Muñoz-Espí, K. Landfester, U. Ziener, Macromolecules 2012, 45, 5108–5113.
- 12B. Marler, U. Oberhagemann, S. Vortmann, H. Gies, Microporous Mater. 1996, 6, 375–383.
- 13
- 13aE. M. Bazzaoui, J. P. Marsault, S. Aeiyach, P. C. Lacaze, Synth. Met. 1994, 66, 217–224;
- 13bS. Garreau, M. Leclerc, N. Errien, G. Louarn, Macromolecules 2003, 36, 692–697.
- 14E. C. Vujanovich, J. W. G. Bloom, S. E. Wheeler, J. Phys. Chem. A 2012, 116, 2997–3003.
- 15H. Yamashita, T. Yumura, J. Phys. Chem. C 2012, 116, 9681–9690.
- 16S. Brückner, W. Porzio, Makromol. Chem. 1988, 189, 961–967.
- 171⊃PTh⋅30 showed the emission peak around 680 nm; however, it was very weak (quantum yield <0.1 %), probably because of the concentration self-quenching (Supporting Information, Figure S11).
- 18
- 18aJ. J. Apperloo, R. A. J. Janssen, P. R. L. Malenfant, J. M. J. Fréchet, Macromolecules 2000, 33, 7038–7043;
- 18bT. Fukuda, Y. Inoue, T. Koga, M. Matsuoka, Y. Miura, Chem. Lett. 2011, 40, 864–866;
- 18cA. Ruseckas, E. B. Namdas, M. Theander, M. Svensson, A. Yartsev, D. Zigmantas, M. R. Andersson, O. Inganäs, V. Sundström, J. Photochem. Photobiol. A 2001, 144, 3–12.
- 19Y. Noguchi, A. Saeki, T. Fujiwara, S. Yamanaka, M. Kumano, T. Sakurai, N. Matsuyama, M. Nakano, N. Hirao, Y. Ohishi, S. Seki, J. Phys. Chem. B 2015, 119, 7219–7230.
- 20
- 20aA. Saeki, S. Seki, T. Sunagawa, K. Ushida, S. Tagawa, Philos. Mag. 2006, 86, 1261–1276;
- 20bA. Saeki, S. Seki, Y. Koizumi, T. Sunagawa, K. Ushida, S. Tagawa, J. Phys. Chem. B 2005, 109, 10015–10019.
- 21G. R. Hutchison, M. A. Ratner, T. J. Marks, J. Am. Chem. Soc. 2005, 127, 16866–16881.
- 22
- 22aR. Kubo, Rep. Prog. Phys. 1966, 29, 255–284;
- 22bR. Kubo, J. Phys. Soc. Jpn. 1957, 12, 570–586.
- 23
- 23aN. Takeda, J. R. Miller, J. Phys. Chem. B 2012, 116, 14715–14723;
- 23bH. Meier, U. Stalmach, H. Kolshorn, Acta Polym. 1997, 48, 379–384.