Blocking Deprotonation with Retention of Aromaticity in a Plant ent-Copalyl Diphosphate Synthase Leads to Product Rearrangement
Graphical Abstract
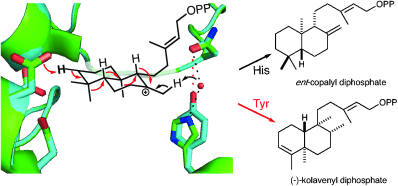
Make the swap: Substitution of histidine, comprising part of the catalytic base group in the ent-copalyl diphosphate synthases, leads to rearrangements. Through a series of 1,2-hydride and methyl shifts of the initially formed bicycle, predominant formation of (−)-kolavenyl diphosphate is observed. Further mutational analysis and quantum chemical calculations provide mechanistic insight into the basis for this profound effect on product outcome.
Abstract
Substitution of a histidine, comprising part of the catalytic base group in the ent-copalyl diphosphate synthases found in all seed plants for gibberellin phytohormone metabolism, by a larger aromatic residue leads to rearrangements. Through a series of 1,2-hydride and methyl shifts of the initially formed bicycle predominant formation of (−)-kolavenyl diphosphate is observed. Further mutational analysis and quantum chemical calculations provide mechanistic insight into the basis for this profound effect on product outcome.