Transfer Hydrosilylation
Graphical Abstract
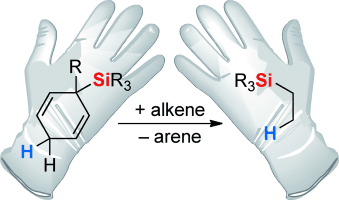
Fair trade: Transfer hydrosilylation is a technique that, unlike the conceptually related transfer hydrogenation, had not been considered for nearly a century. The recently developed radical and ionic transition-metal-free variants rely on aromatization of a cyclohexa-1,4-diene during release of a silicon radical and cation, respectively. Subsequent reaction with typical unsaturated substrates (e.g. alkenes) terminates the transfer process (see scheme).
Abstract
Transfer hydrogenation is without question a common technology in industry and academia. Unlike its countless varieties, conceptually related transfer hydrosilylations had essentially been unreported until the recent development of a radical and an ionic variant. The new methods are both based on a silicon-substituted cyclohexa-1,4-diene and hinge on the aromatization of the corresponding cyclohexadienyl radical and cation intermediates, respectively, concomitant with homo- or heterolytic fission of the SiC bond. Both the radical and ionic transfer hydrosilylation are brought into context with one other in this Minireview, and early insight into the possibility of transfer hydrosilylation is included. Although the current state-of-the-art is certainly still limited, the recent advances have already revealed the promising potential of transfer hydrosilylation.
1. Concept and Strategy
A naive approach to understanding the chemistry of dihydrogen is to look at hydrosilanes. An extreme view is that the silicon atom is nothing but a “fat” hydrogen atom. Although this is clearly a gross oversimplification, the SiH bond often serves as a useful model of the stronger HH bond, particularly in cases where both participate in the same net reaction.
A core area of the dihydrogen arena without a counterpart in silicon chemistry is transfer hydrogenation. Known for more than a century, both heterogeneous and homogeneous methods have found broad application in industrial and academic settings.1 Conversely, conceptually related transfer hydrosilylation processes had remained largely unknown until the research groups of Studer and Oestreich independently disclosed radical and ionic transfer hydrosilylations. Both techniques are based on silicon-substituted cyclohexa-1,4-dienes and exploit aromatization as the driving force (Scheme 1). The stepwise release of the hydrosilane provides equivalents of homolytically (I→II→III, top) or heterolytically (IV→V→VI, bottom) cleaved SiH bonds. The radical sequence is initiated by hydrogen atom abstraction from the methylene group in I (I→II) followed by fragmentation of II (II→III).2 The ionic pathway consists of hydride abstraction from IV to yield the silicon-stabilized cyclohexadienyl cation V3 (IV→V) and formal dissociation of the silicon cation (V→VI). As opposed to the heterolytic cleavage of the SiC bond (V→VI), substitution at the silicon-bearing carbon atom in cyclohexadienyl radical II is required for selective SiC bond homolysis (II→III) because otherwise release of a hydrogen atom competes.4
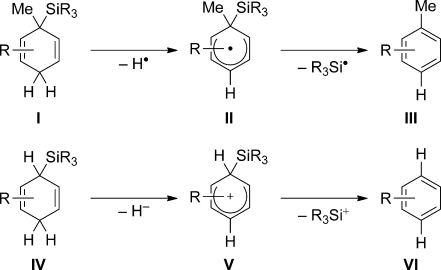
Silicon-substituted cyclohexa-1,4-dienes as hydrosilane surrogates in radical and ionic sequences.
The strategy outlined above is the basis for the development of radical as well as ionic transfer hydrosilylations. This Minireview discusses and compares both approaches and highlights experimental findings that have gone almost unnoticed.
2. Radical Transfer Hydrosilylation
Studer and co-workers introduced and established silicon-substituted cyclohexa-1,4-dienes of type VII as radical chain reducing reagents (Scheme 2).5 As an alternative to conventional tin hydrides, reagents VII enable various reductive defunctionalizations.2, 5, 6 Amrein and Studer also realized that unsaturated radical acceptors such as X would allow the formal transfer of a hydrosilane from the cyclohexa-1,4-diene VII to the π-system of X (Scheme 2).7, 8 After initiation, the cyclohexadienyl radical VIII transfers the silicon fragment to acceptor X (VII→VIII→IX) to yield β-silicon-substituted radical XI. XI then acts as the chain carrier, abstracting a hydrogen atom from VII (VII→VIII) concomitant with formation of the hydrosilylated acceptor XII (XI→XII).
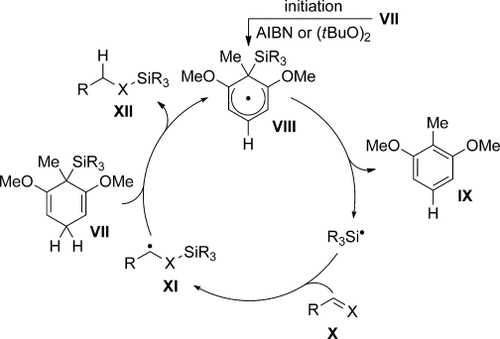
Radical chain of the radical transfer hydrosilylation. X=CH2 (alkenes) and O (aldehydes). AIBN=azobisisobutyronitrile.
Representative examples of VII are resorcinol-derived cyclohexa-1,4-dienes 1–3 with trialkylsilyl or heteroatom-substituted dialkylsilyl groups; 4 is an attractive surrogate for gaseous Me3SiH (Figure 1). As mentioned above, the methyl group at the silicon-bearing carbon atom in 1–4 is necessary to sustain the chain reaction.2 Aryl substituents at the silicon atom are also tolerated (not shown).
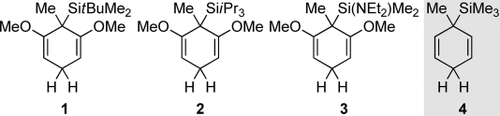
Typical silicon-substituted cyclohexa-1,4-dienes for radical transfer hydrosilylation.
The use of 1 (1.5 equiv) as a transfer reagent is illustrated for a few selected examples (Scheme 3).7 Both terminal and internal alkenes react in decent yields (5→6 and 7→8). The acetate group is compatible with the radical process but will be too Lewis-basic for the ionic variant (see below). The 1,1-disubstituted double bond in β-pinene also accepts the silicon radical, but the initially formed radical intermediate undergoes fast ring opening prior to reduction with 1 (9→10). These transformations are the seminal examples of transfer hydrosilylation. The strength of this process is that it brings about radical hydrosilylation, which usually fails with trialkylsilanes as a result of their relatively strong SiH bonds compared to, for example, (Me3Si)3SiH.
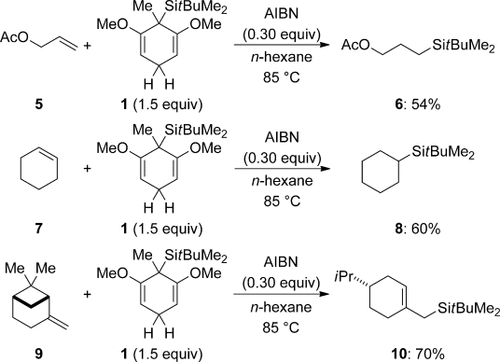
Radical transfer hydrosilylation of alkenes.
Radical transfer hydrosilylation coupled with 5-exo-trig cyclizations is shown for different transfer reagents in Scheme 4.7, 8 The level of diastereocontrol is generally low, but the slight preference for the cis relative configuration is explained with the Beckwith–Houk model for these ring closures. The transfer of tBuMe2SiH released from 1 proceeds under the previously employed setup (AIBN at 85 °C; 11→12). However, the reaction of a twofold excess of Me3SiH surrogate 4 required harsher reaction conditions [(tBuO)2 at 140 °C; 11→13)]. It is noteworthy that even the transfer of Tamao’s (Et2N)Me2SiH from 3 works in reasonable yield, thereby providing a handle for further functional group manipulations (11→14 after treatment with isopropanol). Selected triple bonds also participated in the radical transfer hydrosilylation (not shown).8
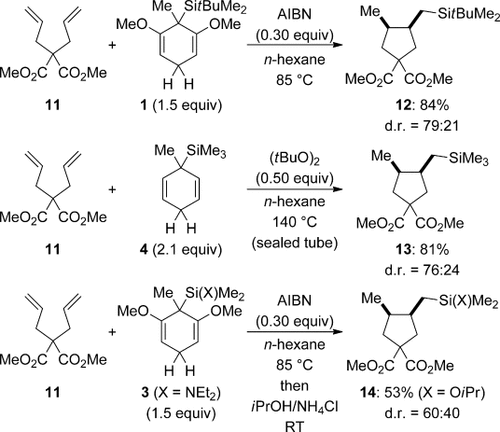
Radical transfer hydrosilylation coupled with radical cyclization.
As part of their investigations, Amrein and Studer demonstrated the possibility of radical transfer hydrosilylation of aldehydes (Scheme 5).8 These reactions had to be performed at 140 °C in a sealed tube with di-tert-butyl peroxide as initiator. Alkyl aldehydes 15 and even cyclohexanone (not shown) reacted readily under these conditions (15→16). Conversely, aryl aldehydes 17, for example, benzaldehyde, were not converted into 18, presumably because of the stability of the intermediate benzyl radical, which cannot be reduced by cyclohexa-1,4-diene 1.
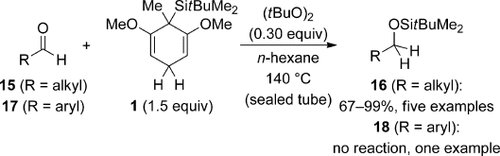
Radical transfer hydrosilylation of aldehydes.
3. Ionic Transfer Hydrosilylation
The origin of ionic transfer hydrosilylation can be seen in the B(C6F5)3-promoted hydride abstraction from 1,4-dihydropyridines (Scheme 6). Stephan, Crudden, and co-workers systematically investigated the abstraction of hydride from Hantzsch-type dihydropyridines by the strong Lewis acid B(C6F5)3.9 Clean formation of the pyridinium ion with [HB(C6F5)3]− as counteranion was found for Hantzsch hydride donors with an NMe rather than a free NH group (19→20, Scheme 6, top). An experiment hidden in publications by Nikonov and co-workers is even more pertinent to ionic transfer hydrosilylation.10 Hydride abstraction from N-silylated 1,4-dihydropyridine 21 generated the ion pair 22 (Scheme 6, bottom). Adduct 22 is a (reversible) frustrated Lewis pair/hydrosilane system11 composed of pyridine/ B(C6F5)3 and Me2PhSiH, from which the hydrosilane is slowly released (22→23, Scheme 6, bottom).

Hydride abstraction from 1,4-dihydropyridines by B(C6F5)3.
Nikonov’s experiment is intriguing in the sense that the electron-deficient borane employed for the hydrosilane release from the partially reduced silicon-substituted hetarene is also a superb Lewis acid for the activation of SiH bonds.12 A broad spectrum of hydrosilylation reactions is in fact catalyzed by B(C6F5)3.13 However, pyridine and B(C6F5)3 form Lewis pair 23, which will hamper the subsequent SiH bond activation. This obstacle would be overcome by using 3-silylated cyclohexa-1,4-dienes instead of N-silylated 1,4-dihydropyridines. The arene formed as waste in the hydrosilane release step would not interfere with B(C6F5)3-catalyzed hydrosilylations. The overall strategy, therefore, consists of two consecutive catalytic cycles, both promoted by B(C6F5)3 (Scheme 7).14 A quantum-chemical treatment by Sakata and Fujimoto later confirmed the mechanism proposed for π-basic alkenes by Simonneau and Oestreich.15 It still needs to be verified whether the concecutive process also applies to σ-basic aldehydes as these could competitively capture the silicon electrophile at an earlier stage that is interrupting the hydrosilane-release cycle.
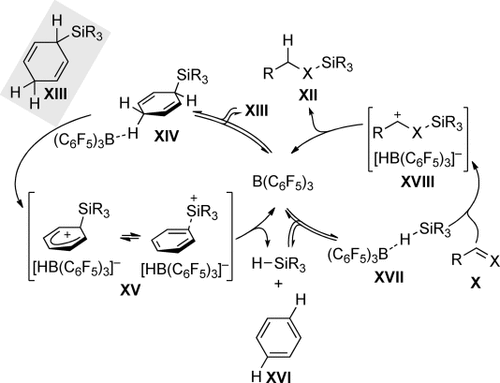
Consecutive catalytic cycles of ionic transfer hydrosilylation. X=CH2 (alkenes) and O (aldehydes).
The hydrosilane-release cycle (Scheme 7, left) commences with coordination of B(C6F5)3 to the methylene CH group opposite to the face with the silicon group (XIII→XIV).15 This reversible interaction eventually leads to hydride abstraction and formation of silicon-stabilized cyclohexadienyl cation XV (XIV→XV).3 Intermediate XV was our source of inspiration, as we had initially been interested in making arene-stabilized silicon cations such as XV by hydride abstraction from cyclohexa-2,5-dien-1-yl-substituted silanes XIII with the trityl cation (the “cyclohexadienyl-leaving-group” approach).16 Neutral B(C6F5)3 yields, however, [HB(C6F5)3]−, which reduces the silicon cation in ion pair XV to afford the hydrosilane and arene XVI. That hydrosilane then enters the hydrosilylation cycle (Scheme 7, right) to form adduct XVII12 in equilibrium. The B(C6F5)3-activated hydrosilane XVII reacts with various π- and σ-basic substrates X, for example, alkenes17 and aldehydes18. Formal transfer of the silicon cation onto the Lewis base is followed by borohydride reduction (X→XVIII→XII).
To test this above strategy, several cyclohexa-1,4-diene-based hydrosilane surrogates were prepared, for example, 24 and 25 (Figure 2, left).14, 19 Variation of the substitution pattern at the silicon atom was also investigated, but the focus of Oestreich and co-workers was on the transfer of otherwise gaseous hydrosilanes such as Me3SiH, Me2SiH2, and difficult-to-handle SiH4. 26 and 27 are such surrogates for monosilane (Figure 2, right).20
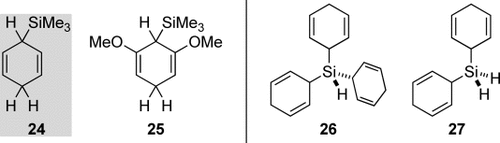
Silicon-substituted cyclohexa-1,4-dienes for ionic transfer hydrosilylation.
The ionic transfer hydrosilylation emerged as broadly applicable to unfunctionalized alkenes (Scheme 8).14 The reaction conditions were mild, just maintaining the reactants together with the catalyst in CH2Cl215 at room temperature. Terminal, that is, mono- and 1,1-disubstituted, alkenes reacted smoothly (→28–33), as did internal alkenes (→34–38). The exo selectivity seen for norbornene (→36) and the predominant cis diastereoselectivity in the hydrosilylation of 1-methylcyclohexene (→37) were evidence of the involvement of carbenium ion intermediates. The hydrosilylation of another trisubstituted alkene, 2-methyl-1H-indene, showcased the better stabilization of a benzylic compared to a tertiary carbenium ion (→38).
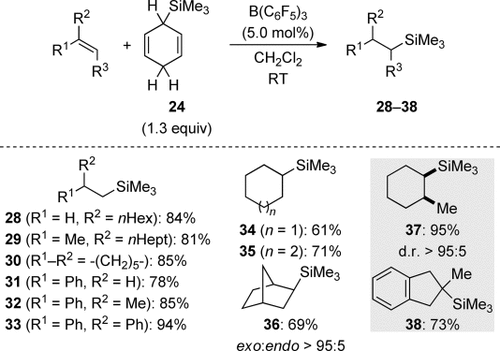
Ionic transfer hydrosilylation of alkenes.
Oestreich and co-workers recently reported a systematic study of the ionic transfer hydrosilylation.19, 21 This work includes screenings of representative π- and σ-donating substrates, electronically and sterically modified surrogates, and partially or fully fluorinated triarylboranes. Selected representative data are depicted in Scheme 9. The reduction of σ-basic acetophenone (40) using standard 24 requires higher temperature than that of π-basic 1,1-diphenylethylene (39) because 40 forms a stronger Lewis acid/base adduct with B(C6F5)3 (column 1). Cognate 4, previously used by Studer and co-workers in the radical transfer hydrosilylation,7 with an additional methyl group at the silicon-bearing carbon atom is less reactive (column 2). Resorcinol-derived 25, which mimics Studer’s reagents (cf. Figure 1), is far more reactive than 24 because of its enhanced hydricity (column 3). However, the Lewis-basic methoxy groups in 25 compete with the substrate for the transfer of the silicon electrophile (cf. XVII→XVIII, Scheme 7). The π-basic alkene is not sufficiently nucleophilic, and demethylation of the resorcinol dimethyl ether was observed. Conversely, the carbonyl hydrosilylation was complete at room temperature within minutes.
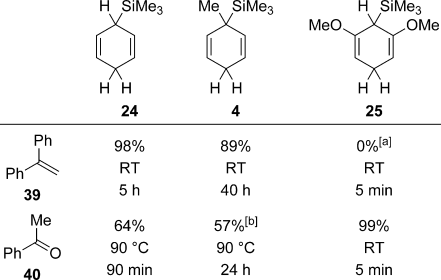
Gauging the reactivity of surrogate/substrate combinations in ionic transfer hydrosilylation. [a] 25 completely consumed. [b] Partial deoxygenation to styrene.
A crucial test of transfer hydrosilylation is whether it would enable the transfer of monosilane. The serious safety issues associated with handling SiH4 have deterred synthetic chemists from its use, and conventional hydrosilylation with this dangerous gas is barely researched. Simonneau and Oestreich introduced solid 26 and liquid 27 as monosilane surrogates to ionic transfer hydrosilylation (Figure 2, right and Scheme 10).20 It was shown that B(C6F5)3 unleashes SiH4 prior to n-fold hydrosilylation of typical alkenes. The chemoselectivity is determined by steric demand and cannot be controlled by the ratio of the reactants. In one case, the use of 27 (0.35 equiv) or 26 (0.55 equiv) led to the selective formation of monohydrosilane 42 (n=3) and dihydrosilane 43 (n=2), respectively (gray box). An α-olefin afforded the tetraorganosilane (→47), but the other transfer reactions yielded either mono- or dihydrosilanes with synthetically useful selectivities, including styrene (→41).
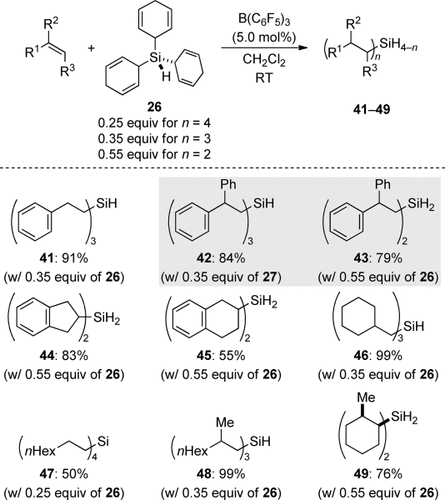
Ionic transfer hydrosilylation of alkenes with monosilane.
4. Outlook
Transfer hydrosilylation is at the early stages of development, but the variants disclosed by the groups of Studer7, 8 and Oestreich14, 19, 20 have already indicated its potential. The radical process is superior to B(C6F5)3 catalysis because of its compatibility with Lewis-basic functional groups, carboxyl groups in particular. Moreover, the ionic pathway cannot transfer silicon groups with bulky substituents, for example, tBuMe2Si and iPr3Si.14 The reaction temperature of the cationic process is, however, an advantage when transferring gaseous and hence difficult-to-handle hydrosilanes, for example, Me3SiH, Me2SiH2, and SiH4. The focus of the work of Simonneau and Oestreich is on these pyrophoric and explosive hydrosilanes, and the monosilane surrogates finally allow safe synthetic chemistry with this hazardous smallest member of the hydrosilane family.
These transfer hydrosilylations are transition-metal free, and this naturally raises the question whether transition metals are also able to catalyze the transfer of a hydrosilane from one molecule to another. Reversibility of the hydrosilylation would be a basic requirement for this. It was again the groups of Nikonov10 and later Oestreich22 who demonstrated such reversibility for 1,4-selective pyridine hydrosilylation (Scheme 11). Nikonov’s fundamental discovery is again buried in his method-oriented publications (cf. Scheme 6, bottom). A ruthenium catalyst promotes hydrosilane transfer from an N-silylated 1,4-dihydropyridine to 3,5-lutidine (21→52, Scheme 11); a nitrile also served as an acceptor (not shown).10 This proof of concept of a transition-metal-catalyzed transfer hydrosilylation could prove viable in the future.
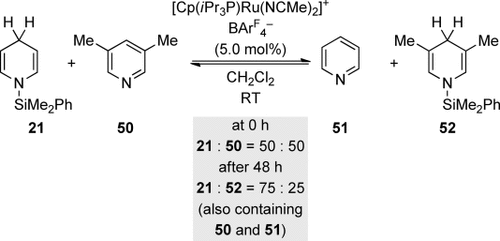
Reversible hydrosilylation of pyridines: An example of a transition-metal-catalyzed transfer hydrosilylation. BArF4−=tetrakis[3,5-bis(trifluoromethyl)phenyl]borate.
Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft (Oe 249/11-1). M.O. is indebted to the Einstein Foundation (Berlin) for an endowed professorship. I thank Dr. Antoine Simonneau and Sebastian Keeß for their enthusiasm and commitment.
Dedicated to Professor Siegfried Blechert on the occasion of his 70th birthday
Biographical Information
Martin Oestreich (born in 1971 in Pforzheim/Germany) is Professor of Organic Chemistry at the Technische Universität Berlin. He received his diploma with Paul Knochel (Marburg, 1996) and his PhD with Dieter Hoppe (Münster, 1999). After a two-year postdoctoral stint with Larry E. Overman (Irvine, 1999–2001), he completed his habilitation with Reinhard Brückner (Freiburg, 2001–2005) and was appointed Professor of Organic Chemistry at the Westfälische Wilhelms-Universität Münster (2006–2011). He also held visiting positions at Cardiff University in Wales (2005) and at The Australian National University in Canberra (2010).