Gold-Catalyzed CH Annulation of Anthranils with Alkynes: A Facile, Flexible, and Atom-Economical Synthesis of Unprotected 7-Acylindoles
Hongming Jin
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorLong Huang
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorJin Xie
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorMatthias Rudolph
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorFrank Rominger
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorCorresponding Author
A. Stephen K. Hashmi
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)Search for more papers by this authorHongming Jin
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorLong Huang
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorJin Xie
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorMatthias Rudolph
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorFrank Rominger
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorCorresponding Author
A. Stephen K. Hashmi
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
Institut für Organische Chemie, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)Search for more papers by this authorGraphical Abstract
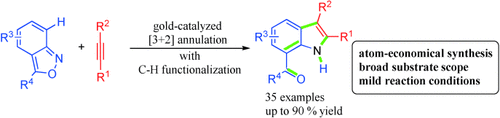
Good as gold: The gold-catalyzed CH annulation of anthranil derivatives with alkynes offers a facile, flexible, and atom-economical one-step route to unprotected 7-acylindoles. The reaction proceeds via an α-imino gold carbene intermediate, which promotes ortho-aryl CH functionalization to afford the product. The transformation proceeds with a broad range of substrates under mild conditions.
Abstract
The gold-catalyzed CH annulation of anthranil derivatives with alkynes offers a facile, flexible, and atom-economical one-step route to unprotected 7-acylindoles. An intermediate α-imino gold carbene, generated by an intermolecular reaction, promotes ortho-aryl CH functionalization to afford the target products. The transformation proceeds with a broad range of substrates under mild conditions. Moreover, the obtained functionalized indole products represent a versatile platform for the construction of diverse indolyl frameworks.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201508309_sm_miscellaneous_information.pdf2.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. J. Sundberg in The Chemistry of Indoles, Academic Press, New York, 1970;
- 1bJ. E. Saxton, Nat. Prod. Rep. 1997, 14, 559;
- 1cQ. Ye, Y.-H. Li, Y.-M. Song, X.-F. Huang, R.-G. Xiong, Z. Xue, Inorg. Chem. 2005, 44, 3618;
- 1dJ. Landwehr, S. George, E.-M. Karg, D. Poeckel, D. Steinhilber, R. Troschuetz, O. Werz, J. Med. Chem. 2006, 49, 4327;
- 1eW. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z.-S. Wang, H. Tian, Adv. Funct. Mater. 2011, 21, 756;
- 1fM. Inman, C. J. Moody, Chem. Sci. 2013, 4, 29.
- 2
- 2aM. P. Moyer, J. F. Shiurba, H. Rapoport, J. Org. Chem. 1986, 51, 5106;
- 2bF. D. Monache, R. D. Benedetto, M. A. De Moraes e Souza, P. Sandor, Gazz. Chim. Ital. 1990, 120, 387;
- 2cA. B. Smith III, L. Kürti, A. H. Davulcu, Org. Lett. 2006, 8, 2167;
- 2dT. Barf, F. Lahmann, K. Hammer, S. Haile, E. Axen, C. Medina, J. Uppenberg, S. Svensson, L. Rondahl, T. Lundback, Bioorg. Med. Chem. Lett. 2009, 19, 1745;
- 2eJ. A. Nieman, S. K. Nair, S. E. Heasley, B. L. Schultz, H. M. Zerth, R. A. Nugent, K. Chen, K. J. Stephanski, T. A. Hopkins, M. L. Knechtel, N. L. Oien, J. L. Wieber, M. W. Wathen, Bioorg. Med. Chem. Lett. 2010, 20, 3039.
- 3
- 3aE. Fischer, F. Jourdan, Ber. Dtsch. Chem. Ges. 1883, 16, 2241;
10.1002/cber.188301602141 Google Scholar
- 3bG. Bartoli, F. Palmieri, M. Bosco, R. Dalpozzo, Tetrahedron Lett. 1989, 30, 2129;
- 3cR. C. Larock, E. K. Yum, J. Am. Chem. Soc. 1991, 113, 6689;
- 3dG. Zeni, R. C. Larock, Chem. Rev. 2004, 104, 2285.
- 4Recent transition-metal-catalyzed syntheses of indoles:
- 4aS. Cacchi, G. Fabrizi, Chem. Rev. 2005, 105, 2873;
- 4bM. Bandini, A. Eichholzer, Angew. Chem. Int. Ed. 2009, 48, 9608; Angew. Chem. 2009, 121, 9786;
- 4cD. Shu, W. Song, X. Li, W. Tang, Angew. Chem. Int. Ed. 2013, 52, 3237; Angew. Chem. 2013, 125, 3319;
- 4dB. Liu, C. Song, C. Sun, S. Zhou, J. Zhu, J. Am. Chem. Soc. 2013, 135, 16625;
- 4eD. Shan, Y. Gao, Y. Jia, Angew. Chem. Int. Ed. 2013, 52, 4902; Angew. Chem. 2013, 125, 5002;
- 4fJ. S. Alford, J. E. Spangler, H. M. L. Davies, J. Am. Chem. Soc. 2013, 135, 11712;
- 4gC. Wang, H. Sun, Y. Fang, Y. Huang, Angew. Chem. Int. Ed. 2013, 52, 5795; Angew. Chem. 2013, 125, 5907;
- 4hD. Zhao, Z. Shi, F. Glorius, Angew. Chem. Int. Ed. 2013, 52, 12426; Angew. Chem. 2013, 125, 12652;
- 4iL. Zheng, R. Hua, Chem. Eur. J. 2014, 20, 2352;
- 4jG. Zhang, H. Yu, G. Qin, H. Huang, Chem. Commun. 2014, 50, 4331;
- 4kS. E. Kiruthika, P. T. Perumal, Org. Lett. 2014, 16, 484;
- 4lS. G. Dawande, V. Kanchupalli, J. Kalepu, H. Chennamsetti, B. S. Lad, S. Katukojvala, Angew. Chem. Int. Ed. 2014, 53, 4076; Angew. Chem. 2014, 126, 4160;
- 4mC. Jones, Q. Nguyen, T. G. Driver, Angew. Chem. Int. Ed. 2014, 53, 785; Angew. Chem. 2014, 126, 804;
- 4nT. Miura, Y. Funakoshi, M. Murakami, J. Am. Chem. Soc. 2014, 136, 2272;
- 4oU. Sharma, R. Kancherla, T. Naveen, S. Agasti, D. Maiti, Angew. Chem. Int. Ed. 2014, 53, 11895; Angew. Chem. 2014, 126, 12089;
- 4pN. Jana, F. Zhou, T. G. Driver, J. Am. Chem. Soc. 2015, 137, 6738.
- 5Selected reviews on gold catalysis:
- 5aA. S. K. Hashmi, Chem. Rev. 2007, 107, 3180;
- 5bD. J. Gorin, F. D. Toste, Nature 2007, 446, 395;
- 5cZ. Li, C. Brouwer, C. He, Chem. Rev. 2008, 108, 3239;
- 5dA. Arcadi, Chem. Rev. 2008, 108, 3266;
- 5eM. Rudolph, A. S. K. Hashmi, Chem. Soc. Rev. 2012, 41, 2448;
- 5fA. S. K. Hashmi, Acc. Chem. Res. 2014, 47, 864;
- 5gH.-S. Yeom, S. Shin, Acc. Chem. Res. 2014, 47, 966;
- 5hL. Zhang, Acc. Chem. Res. 2014, 47, 877.
- 6Recent examples of gold-catalyzed intramolecluar nitrene transfer for the synthesis of indolyl and pseudoindoxyl:
- 6aA. Wetzel, F. Gagosz, Angew. Chem. Int. Ed. 2011, 50, 7354; Angew. Chem. 2011, 123, 7492;
- 6bB. Lu, Y. Luo, L. Liu, L. Ye, Y. Wang, L. Zhang, Angew. Chem. Int. Ed. 2011, 50, 8358; Angew. Chem. 2011, 123, 8508;
- 6cN. Li, T.-Y. Wang, L.-Z. Gong, L. Zhang, Chem. Eur. J. 2015, 21, 3585;
- 6dC.-H. Shen, Y. Pan, Y.-F. Yu, Z.-S. Wang, W. He, T. Li, L.-W. Ye, J. Organomet. Chem. 2015, 795, 63.
- 7Gold-catalyzed intermolecular method for the construction of heterocyclic frameworks:
- 7aP. W. Davies, A. Cremonesi, L. Dumitrescu, Angew. Chem. Int. Ed. 2011, 50, 8931; Angew. Chem. 2011, 123, 9093;
- 7bE. Chatzopoulou, P. W. Davies, Chem. Commun. 2013, 49, 8617;
- 7cA.-H. Zhou, Q. He, C. Shu, Y.-F. Yu, S. Liu, T. Zhao, W. Zhang, X. Lu, L.-W. Ye, Chem. Sci. 2015, 6, 1265;
- 7dC. Shu, Y.-H. Wang, B. Zhou, X.-L. Li, Y.-F. Ping, X. Lu, L.-W. Ye, J. Am. Chem. Soc. 2015, 137, 9567;
- 7eY. Wang, L. Liu, L. Zhang, Chem. Sci. 2013, 4, 739.
- 8
- 8aW. Yang, T. Wang, Y. Yu, S. Shi, T. Zhang, A. S. K. Hashmi, Adv. Synth. Catal. 2013, 355, 1523–1528;
- 8bA. S. K. Hashmi, W. Yang, F. Rominger, Chem. Eur. J. 2012, 18, 6576–6580;
- 8cT. Wang, S. Shi, D. Pflästerer, E. Rettenmeier, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 292.
- 9
- 9aG. Evano, A. Coste, K. Jouvin, Angew. Chem. Int. Ed. 2010, 49, 2840; Angew. Chem. 2010, 122, 2902;
- 9bE. Rettenmeier, A. M. Schuster, M. Rudolph, F. Rominger, C. Gade, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2013, 52, 5880; Angew. Chem. 2013, 125, 5993;
- 9cX.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski, R. P. Hsung, Acc. Chem. Res. 2014, 47, 560.
- 10CCDC 1415770 (3 q) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 11M. Najafi, Monatsh. Chem. 2014, 145, 291.
- 12For details, see Table S1 in the Supporting Information.
- 13D. S. Black, N. Kumar, P. S. R. Mitchell, J. Org. Chem. 2002, 67, 2464.
- 14
- 14aD. S. C. Black, A. J. Ivory, P. A. Keller, N. Kumar, Synthesis 1989, 322;
- 14bM. Adib, M. H. Sayahia, Monatsh. Chem. 2006, 137, 207;
- 14cH. McNab, D. J. Nelson, E. J. Rozgowska, Synthesis 2009, 2171;
- 14dL. Yin, S. Lucas, F. U. Kazmaier, Q. Hu, R. W. Hartmann, J. Med. Chem. 2012, 55, 6629.