Organocatalytic Asymmetric 1,6-Addition/1,4-Addition Sequence to 2,4-Dienals for the Synthesis of Chiral Chromans†
This work was made possible by support from Aarhus University and Carlsberg Foundation. K.S.F. and B.M.P. thank the CAPES Brazilian Foundation (BEX 14224/13-5 and BEX 952513-0) for financial support. We also thank Magnus E. Jensen for performing X-ray analysis.
Graphical Abstract
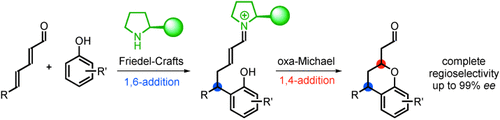
One six one four: Based on a 1,6-Friedel–Crafts/1,4-oxa-Michael sequence, an organocatalyst directs the reaction of hydroxyarenes with a vinylogous iminium-ion intermediate to give only one out of four possible regioisomers. The reaction provides optically active chromans in high yields with up to 99 % ee.
Abstract
A novel asymmetric organocatalytic 1,6-addition/1,4-addition sequence to 2,4-dienals is described. Based on a 1,6-Friedel–Crafts/1,4-oxa-Michael cascade, the organocatalyst directs the reaction of hydroxyarenes with a vinylogous iminium-ion intermediate to give only one out of four possible regioisomers, thus providing optically active chromans in high yields and 94–99 % ee. Furthermore, several transformations are presented, including the formation of an optically active macrocyclic lactam. Finally, the mechanism for the novel reaction is discussed based on computational studies.