Rhodium-Catalyzed Enantioselective Intramolecular CH Silylation for the Syntheses of Planar-Chiral Metallocene Siloles†
Dr. Qing-Wei Zhang
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
Search for more papers by this authorKun An
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
Search for more papers by this authorLi-Chuan Liu
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
Search for more papers by this authorYuan Yue
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei He
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)Search for more papers by this authorDr. Qing-Wei Zhang
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
Search for more papers by this authorKun An
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
Search for more papers by this authorLi-Chuan Liu
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
Search for more papers by this authorYuan Yue
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei He
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)
School of Medicine and Tsinghua-Peking Joint Centers for Life Science, Tsinghua University, Beijing 100084 (China)Search for more papers by this authorWe acknowledge financial support from the National Key Basic Research Program of China (2012CB224802), NSF of China (21371107), Tsinghua-Peking Joint Centers for Life Sciences, and the China Postdoctoral Science Foundation (2013M540083). We thank Prof. Junliang Zhang (East China Normal University) for very helpful suggestions on the syntheses of Segphos ligands and Prof. Wenxiong Zhang (Peking University) for his assistance in solving the X-ray structure of compound 2 ab.
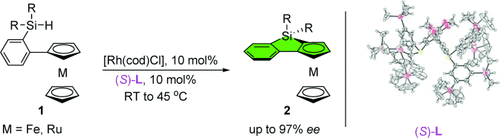
Graphical Abstract
New on the (metallo)cene: The title reaction of cyclopentadiene (Cp) rings in Fe and Ru metallocenes is reported. Fine-tuning the steric hindrance of diphosphine ligands led to the identification of (S)-TMS-Segphos [(S)-L], which enabled efficient, enantioselective CH silylation of the Cp rings in metallocenes under mild reaction conditions. cod=1,5-cyclooctadiene, TMS=trimethylsilyl.
Abstract
Reported herein is the rhodium-catalyzed enantioselective CH bond silylation of the cyclopentadiene rings in Fe and Ru metallocenes. Thus, in the presence of (S)-TMS-Segphos, the reactions took place under very mild conditions to afford metallocene-fused siloles in good to excellent yields and with ee values of up to 97 %. During this study it was observed that the steric hindrance of chiral ligands had a profound influence on the reactivity and enantioselectivity of the reaction, and might hold the key to accomplishing conventionally challenging asymmetric CH silylations.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201502548_sm_miscellaneous_information.pdf7.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. G. de Azevedo, K. P. C. Vollhardt, Synlett 2002, 1019;
- 1b Metallocenes (Eds.: ), VCH, Weinheim, 1998;
- 1c Ferrocenes (Ed.: ), Wiley, Chichester, UK, 2008;
- 1dD. R. van Staveren, N. Metzler-Nolte, Chem. Rev. 2004, 104, 5931.
- 2
- 2aG. C. Fu, Acc. Chem. Res. 2004, 37, 542;
- 2bC. T. Check, K. P. Jang, C. B. Schwamb, A. S. Wong, M. H. Wang, K. A. Scheidt, Angew. Chem. Int. Ed. 2015, 54, 4264; Angew. Chem. 2015, 127 4338;
- 2cL. X. Dai, T. Tu, S. L. You, W. P. Deng, X. L. Hou, Acc. Chem. Res. 2003, 36, 659;
- 2dN. Butt, D. Liu, W. Zhang, Synlett 2014, 615;
- 2eR. Gómez Arrayás, J. Adrio, J. C. Carretero, Angew. Chem. Int. Ed. 2006, 45, 7674; Angew. Chem. 2006, 118, 7836.
- 3
- 3aD. Schaarschmidt, H. Lang, Organometallics 2013, 32, 5668;
- 3bA. Togni, Angew. Chem. Int. Ed. Engl. 1996, 35, 1475; Angew. Chem. 1996, 108, 1581.
- 4
- 4aC. Genet, S. J. Canipa, P. O’Brien, S. Taylor, J. Am. Chem. Soc. 2006, 128, 9336;
- 4bE. P. Kündig, P. D. Chaudhuri, D. House, G. Bernardinelli, Angew. Chem. Int. Ed. 2006, 45, 1092; Angew. Chem. 2006, 118, 1110;
- 4cB. Gotov, H.-G. Schmalz, Org. Lett. 2001, 3, 1753;
- 4dM. Ogasawara, S. Watanabe, K. Nakajima, T. Takahashi, J. Am. Chem. Soc. 2010, 132, 2136;
- 4eM. Murai, J. i. Uenishi, M. Uemura, Org. Lett. 2010, 12, 4788;
- 4fM. Uemura, H. Nishimura, T. Hayashi, Tetrahedron Lett. 1993, 34, 107;
- 4gE. Bergin, D. L. Hughes, C. J. Richards, Tetrahedron: Asymmetry 2010, 21, 1619.
- 5For reviews on asymmetric CH activation, see:
- 5aR. Giri, B. F. Shi, K. M. Engle, N. Maugel, J. Q. Yu, Chem. Soc. Rev. 2009, 38, 3242;
- 5bH. M. Peng, L.-X. Dai, S.-L. You, Angew. Chem. Int. Ed. 2010, 49, 5826; Angew. Chem. 2010, 122, 5962;
- 5cC. Zheng, S.-L. You, RSC Adv. 2014, 4, 6173;
- 5dJ. Wencel-Delord, F. Colobert, Chem. Eur. J. 2013, 19, 14010; for selected examples see:
- 5eB.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. Int. Ed. 2008, 47, 4882; Angew. Chem. 2008, 120, 4960;
- 5fM. R. Albicker, N. Cramer, Angew. Chem. Int. Ed. 2009, 48, 9139; Angew. Chem. 2009, 121, 9303;
- 5gM. Nakanishi, D. Katayev, C. Besnard, E. P. Kündig, Angew. Chem. Int. Ed. 2011, 50, 7438; Angew. Chem. 2011, 123, 7576;
- 5hS. Anas, A. Cordi, H. B. Kagan, Chem. Commun. 2011, 47, 11483;
- 5iR. Shintani, H. Otomo, K. Ota, T. Hayashi, J. Am. Chem. Soc. 2012, 134, 7305;
- 5jN. Martin, C. Pierre, M. Davi, R. Jazzar, O. Baudoin, Chem. Eur. J. 2012, 18, 4480;
- 5kQ. Li, Z.-X. Yu, Angew. Chem. Int. Ed. 2011, 50, 2144; Angew. Chem. 2011, 123, 2192.
- 6S. Siegel, H.-G. Schmalz, Angew. Chem. Int. Ed. Engl. 1997, 36, 2456; Angew. Chem. 1997, 109, 2569.
- 7
- 7aD. W. Gao, Y. C. Shi, Q. Gu, Z. L. Zhao, S. L. You, J. Am. Chem. Soc. 2013, 135, 86;
- 7bY. C. Shi, R. F. Yang, D. W. Gao, S. L. You, Beilstein J. Org. Chem. 2013, 9, 1891.
- 8
- 8aC. Pi, Y. Li, X. Cui, H. Zhang, Y. Han, Y. Wu, Chem. Sci. 2013, 4, 2675;
- 8bC. Pi, X. Cui, X. Liu, M. Guo, H. Zhang, Y. Wu, Org. Lett. 2014, 16, 5164.
- 9
- 9aT. Shibata, T. Shizuno, Angew. Chem. Int. Ed. 2014, 53, 5410; Angew. Chem. 2014, 126, 5514;
- 9bwhile this manuscript is under revision, Shibata et al. published asymmetric CH silylation hinged on Rh/chiral diene catalyst. T. Shibata, T. Shizuno, T. Sasaki, Chem. Commun. 2015, DOI: .
- 10D. W. Gao, Q. Yin, Q. Gu, S. L. You, J. Am. Chem. Soc. 2014, 136, 4841.
- 11
- 11aR. Deng, Y. Huang, X. Ma, G. Li, R. Zhu, B. Wang, Y. B. Kang, Z. Gu, J. Am. Chem. Soc. 2014, 136, 4472;
- 11bX. Ma, Z. Gu, RSC Adv. 2014, 4, 36241.
- 12L. Liu, A. A. Zhang, R. J. Zhao, F. Li, T. J. Meng, N. Ishida, M. Murakami, W. X. Zhao, Org. Lett. 2014, 16, 5336.
- 13
- 13aS. Yamaguchi, K. Tamao, Silicon-containing Polymers: The Science and Technology of their Synthesis and Applications (Eds.: ), Kluwer Academic Publishers, Dordrecht, 2000, pp. 461–498;
- 13bJ. Y. Corey in Advances in Organometallic Chemistry, Vol. 59 (Eds.: ), Academic Press, New York, 2011, pp. 1–328.
- 14For reviews see:
- 14aJ. F. Hartwig, Acc. Chem. Res. 2012, 45, 864; for selected examples see:
- 14bN. Tsukada, J. F. Hartwig, J. Am. Chem. Soc. 2005, 127, 5022;
- 14cE. M. Simmons, J. F. Hartwig, Nature 2012, 483, 70;
- 14dC. Cheng, J. F. Hartwig, Science 2014, 343, 853;
- 14eB. Lu, J. R. Falck, Angew. Chem. Int. Ed. 2008, 47, 7508; Angew. Chem. 2008, 120, 7618;
- 14fH. Ihara, M. Suginome, J. Am. Chem. Soc. 2009, 131, 7502;
- 14gF. Kakiuchi, K. Tsuchiya, M. Matsumoto, E. Mizushima, N. Chatani, J. Am. Chem. Soc. 2004, 126, 12792;
- 14hM. Murata, N. Fukuyama, J.-i. Wada, S. Watanabe, Y. Masuda, Chem. Lett. 2007, 36, 910;
- 14iH. F. Klare, M. Oestreich, J. Ito, H. Nishiyama, Y. Ohki, K. Tatsumi, J. Am. Chem. Soc. 2011, 133, 3312.
- 15
- 15aJ. Oyamada, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2011, 50, 10720; Angew. Chem. 2011, 123, 10908;
- 15bS. Furukawa, J. Kobayashi, T. Kawashima, J. Am. Chem. Soc. 2009, 131, 14192;
- 15cD. Leifert, A. Studer, Org. Lett. 2015, 17, 386.
- 16
- 16aT. Ureshino, T. Yoshida, Y. Kuninobu, K. Takai, J. Am. Chem. Soc. 2010, 132, 14324;
- 16bY. Kuninobu, K. Yamauchi, N. Tamura, T. Seiki, K. Takai, Angew. Chem. Int. Ed. 2013, 52, 1520; Angew. Chem. 2013, 125, 1560.
- 17
- 17aK. Tamao, K. Nakamura, H. Ishii, S. Yamaguchi, M. Shiro, J. Am. Chem. Soc. 1996, 118, 12469;
- 17bT. Hayashi, K. Yamamoto, M. Kumada, Tetrahedron Lett. 1974, 15, 331;
10.1016/S0040-4039(01)82207-3 Google Scholar
- 17cT. Ohta, M. Ito, A. Tsuneto, H. Takaya, J. Chem. Soc. Chem. Commun. 1994, 2525;
- 17dY. Kurihara, M. Nishikawa, Y. Yamanoi, H. Nishihara, Chem. Commun. 2012, 48, 11564;
- 17eK. Igawa, D. Yoshihiro, N. Ichikawa, N. Kokan, K. Tomooka, Angew. Chem. Int. Ed. 2012, 51, 12745; Angew. Chem. 2012, 124, 12917;
- 17fD. R. Schmidt, S. J. O’Malley, J. L. Leighton, J. Am. Chem. Soc. 2003, 125, 1190.
- 18For reviews and recent examples of silole syntheses, see:
- 18aL. Wang, Z. Duan, Chin. Sci. Bull. 2012, 58, 307;
- 18bQ. W. Zhang, K. An, W. He, Angew. Chem. Int. Ed. 2014, 53, 5667; Angew. Chem. 2014, 126, 5773;
- 18cR. Shintani, C. Takagi, T. Ito, M. Naito, K. Nozaki, Angew. Chem. Int. Ed. 2015, 54, 1616; Angew. Chem. 2015, 127, 1636.
- 19M. Berthod, G. Mignani, G. Woodward, M. Lemaire, Chem. Rev. 2005, 105, 1801.
- 20J. H. Xie, Q. L. Zhou, Acc. Chem. Res. 2008, 41, 581.
- 21C. S. Sevov, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 10625.
- 22CCDC 1034947 [(S)-L4 d] and 1054034 [(Sp)-2 ab] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 23Z. Zhang, H. Qian, J. Longmire, X. Zhang, J. Org. Chem. 2000, 65, 6223.
- 24
- 24aM. Raghunath, X. Zhang, Tetrahedron Lett. 2005, 46, 8213;
- 24bM. N. Birkholz née Gensow, Z. Freixa, P. W. van Leeuwen, Chem. Soc. Rev. 2009, 38, 1099;
- 24cF. Liu, D. Qian, L. Li, X. Zhao, J. Zhang, Angew. Chem. Int. Ed. 2010, 49, 6669; Angew. Chem. 2010, 122, 6819; also see ref. [23].