Cu–N Dopants Boost Electron Transfer and Photooxidation Reactions of Carbon Dots†
Wenting Wu
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorLiying Zhan
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorWeiyu Fan
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorJizhong Song
Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorXiaoming Li
Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorZhongtao Li
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorRuiqin Wang
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorJinqiang Zhang
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorJingtang Zheng
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorCorresponding Author
Mingbo Wu
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Mingbo Wu, State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Haibo Zeng, Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Haibo Zeng
Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Mingbo Wu, State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Haibo Zeng, Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorWenting Wu
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorLiying Zhan
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorWeiyu Fan
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorJizhong Song
Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorXiaoming Li
Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorZhongtao Li
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorRuiqin Wang
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorJinqiang Zhang
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorJingtang Zheng
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Search for more papers by this authorCorresponding Author
Mingbo Wu
State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Mingbo Wu, State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Haibo Zeng, Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Haibo Zeng
Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Mingbo Wu, State Key Laboratory of Heavy Oil Processing, School of Chemical Engineering, China University of Petroleum, Qingdao 266580 (China)
Haibo Zeng, Institute of Optoelectronics and Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, 210094 (P.R. China)
Search for more papers by this authorThis work was financially supported by the National Basic Research Program of China (2014CB931700), NSFC (61222403, 21302224, 20876176, 51303212, 51372277, 51172285, and 51303202), China Postdoctoral Science Foundation (2014M560590), Shandong Provincial Natural Science Foundation (ZR2013BQ028 and ZR2013EMQ013), Project of Science and Technology Program for Basic Research of Qingdao (14-2-4-47-jch) and the State Key Laboratory of Fine Chemicals (KF1203).
Graphical Abstract
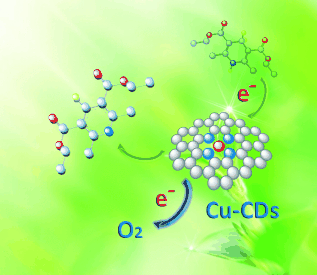
Doped dots: Cu–N-doped carbon dots (Cu-CDs) were fabricated by a one-step pyrolytic synthesis using Na2[Cu(EDTA)] as the precursor. Cu–N dopants concomitantly boost the conductivity and the electron-accepting and -donating abilities of the CDs, enhancing the electron-transfer process in the photooxidation of 1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylate (see picture; H yellow, N blue, C light blue, O red).
Abstract
The broadband light-absorption ability of carbon dots (CDs) has inspired their application in photocatalysis, however this has been impeded by poor electron transfer inside the CDs. Herein, we report the preparation of Cu–N-doped CDs (Cu-CDs) and investigate both the doping-promoted electron transfer and the performance of the CDs in photooxidation reactions. The Cu–N doping was achieved through a one-step pyrolytic synthesis of CDs with Na2[Cu(EDTA)] as precursor. As confirmed by ESR, FTIR, and X-ray photoelectron spectroscopies, the Cu species chelates with the carbon matrix through Cu–N complexes. As a result of the Cu–N doping, the electron-accepting and -donating abilities were enhanced 2.5 and 1.5 times, and the electric conductivity was also increased to 171.8 μs cm−1. As a result of these enhanced properties, the photocatalytic efficiency of CDs in the photooxidation reaction of 1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylate is improved 3.5-fold after CD doping.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501912_sm_miscellaneous_information.pdf886.9 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aB. König in Chemical Photocatalysis, Vol. 7, 8 (Eds.: ), Wiley-VCH, Weinheim, 2013, pp. 111–149;
- 1bY. Yamada, T. Miyahigashi, H. Kotani, K. Ohkubo, S. Fukuzumi, J. Am. Chem. Soc. 2011, 133, 16136;
- 1cA. T. Bell, Science 2003, 299, 1688.
- 2C. Q. Ding, A. W. Zhu, Y. Tian, Acc. Chem. Res. 2014, 47, 20.
- 3L. Cao, M. J. Meziani, S. Sahu, Y. P. Sun, Acc. Chem. Res. 2013, 46, 171.
- 4H. T. Li, Z. H. Kang, Y. Liu, S. T. Lee, J. Mater. Chem. 2012, 22, 24230.
- 5X. M. Li, S. L. Zhang, S. A. Kulinich, Y. L. Liu, H. B. Zeng, Sci. Rep. 2014, 4, 4976.
- 6
- 6aQ. Liu, B. D. Guo, Z. Y. Rao, B. H. Zhang, J. R. Gong, Nano. Lett. 2013, 13, 2436;
- 6bM. Wu, Y. Wang, W. Wu, C. Hu, X. Wang, J. Zheng, Z. Li, B. Jiang, J. Qiu, Carbon 2014, 78, 480.
- 7H. T. Li, R. H. Liu, Y. Liu, H. Huang, H. Yu, H. Ming, S. Y. Lian, S. T. Lee, Z. H. Kang, J. Mater. Chem. 2012, 22, 17470.
- 8X. Zhang, F. Wang, H. Huang, H. T. Li, X. Han, Y. Liu, Z. H. Kang, Nanoscale 2013, 5, 2274.
- 9W. T. Wu, L. Y. Zhan, K. Ohkubo, Y. Yamada, M. B. Wu, S. Fukuzumi, J. Photochem. Photobiol. B 2014, DOI: .
- 10F. Wang, M. Kreiter, B. He, S. P. Pang, C. Y. Liu, Chem. Commun. 2010, 46, 3309.
- 11S. Chandra, P. Patra, S. H. Pathan, S. Roy, S. Mitra, A. Layek, R. Bhar, P. Pramanik, A. Goswami, J. Mater. Chem. B 2013, 1, 2375.
- 12X. M. Li, Y. L. Liu, X. F. Song, H. Wang, H. S. Gu, H. B. Zeng, Angew. Chem. Int. Ed. 2015, 54, 1759; Angew. Chem. 2015, 127, 1779.
- 13J. Ge, M. Lan, B. Zhou, W. Liu, L. Guo, H. Wang, Q. Jia, G. Niu, X. Huang, H. Zhou, X. Meng, P. Wang, C. Lee, W. Zhang, X. Han, Nat. Commun. 2014, 5, 4596.
- 14R. H. Liu, H. Huang, H. T. Li, Y. Liu, J. Zhong, Y. Y. Li, S. Zhang, Z. H. Kang, ACS Catal. 2014, 4, 328.
- 15N. Y. Liu, J. Liu, W. Q. Kong, H. Li, H. Huang, Y. Liu, Z. H. Kang, J. Mater. Chem. B 2014, 2, 5768.
- 16
- 16aJ. Liu, H. C. Zhang, D. Tang, X. Zhang, L. K. Yan, Y. Z. Han, H. Huang, Y. Liu, Z. H. Kang, ChemCatChem 2014, 6, 2634;
- 16bJ. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S.-T. Lee, J. Zhong, Z. Kang, Science 2015, 347, 6225.
- 17J. Q. Pan, Y. Z. Sheng, J. X. Zhang, J. M. Wei, P. Huang, X. Zhang, B. X. Feng, J. Mater. Chem. A 2014, 2, 18082.
- 18H. Ming, Z. Ma, Y. Liu, K. M. Pan, H. Yu, F. Wang, Z. H. Kang, Dalton Trans. 2012, 41, 9526.
- 19Y. Guo, B. X. Li, Carbon 2015, 82, 459.
- 20
- 20aR. G. Reddy, S. Balasubramanian, K. Chennakesavulu, J. Mater. Chem. A 2014, 2, 15598;
- 20bC. Balzani, S. Campagna in Topics in Current Chemistry, Vol. 280 (Eds.: ), Springer, Berlin, 2007, pp. 70–110.
- 21A. Poater, M. Sola, Beilstein J. Org. Chem. 2013, 9, 585.
- 22K. D. Karlin, S. Kaderli, A. Zuberbuhler, Acc. Chem. Res. 1997, 30, 139.
- 23V. S. I. Sprakel, M. C. Feiters, W. M. Klaucke, M. Klopstra, J. Brinksma, B. L. Feringa, K. D. Karlin, R. J. M. Nolte, Dalton Trans. 2005, 3522.
- 24D. Zhang, L. Wu, L. Zhou, X. Han, Q. Yang, L. Zhang, C. Tung, J. Am. Chem. Soc. 2004, 126, 3440.
- 25G. Merza, B. László, A. Oszkó, G. Pótári, K. Baán, A. Erdőhelyi, J. Mol. Catal. A 2014, 393, 117.
- 26J. L. Yang, X. P. Shen, Z. Y. Ji, H. Zhou, G. X. Zhu, K. M. Chen, Appl. Surf. Sci. 2014, 316, 575.
- 27Z. H. Kang, E. B. Wang, B. D. Mao, Z. M. Su, L. Gao, S. Y. Lian, L. Xu, J. Am. Chem. Soc. 2005, 127, 6534.
- 28N. Liu, F. Luo, H. X. Wu, Y. H. Liu, C. Zhang, J. Chen, Adv. Funct. Mater. 2008, 18, 1518.
- 29J. Lu, J. X. Yang, J. Z. Wang, A. Lim, S. Wang, K. P. Loh, ACS Nano 2009, 3, 2367.
- 30Z. T. Luo, Y. Lu, L. A. Somers, A. T. C. Johnson, J. Am. Chem. Soc. 2009, 131, 898.
- 31G. Eda, Y. Y. Lin, C. Mattevi, H. Yamaguchi, H. A. Chen, I. S. Chen, C. W. Chen, M. Chhowalla, Adv. Mater. 2010, 22, 505.
- 32M. Röckert, M. Franke, Q. Tariq, S. Ditze, M. Stark, P. Uffinger, D. Wechsler, U. Singh, J. Xiao, H. Marbach, H. P. Steinrück, O. Lytken, Chem. Eur. J. 2014, 20, 8948.
- 33J. J. Brege, C. E. Hamilton, C. A. Crouse, A. R. Barron, Nano Lett. 2009, 9, 2239.
- 34P. Gayathri, K. A. Senthil, Langmuir 2014, 30, 10513.
- 35T. A. Mohamed, I. A. Shaaban, R. S. Farag, W. M. Zoghaib, M. S. Afifi, Spectrochim. Acta Part A 2015, 135, 417.
- 36J. Xia, S. Yuan, Z. Wang, S. Kirklin, B. Dorney, D. J. Liu, L. Yu, Macromolecules 2010, 43, 3325.
- 37F. Y. Chen, X. Zhao, H. J. Liu, J. H. Qu, Appl. Catal. B 2014, 158, 85.
- 38X. Wang, L. Cao, F. S. Lu, M. J. Meziani, H. T. Li, G. Qi, B. Zhou, B. A. Harruff, F. Kermarreca, Y. P. Sun, Chem. Commun. 2009, 3774.
- 39Z. T. Li, G. L. Wu, D. Liu, W. T. Wu, B. Jiang, J. T. Zheng, Y. P. Li, J. H. Li, M. B. Wu, J. Mater. Chem. A 2014, 2, 7471.
- 40X. B. Lu, X. Wang, J. Jin, Q. Zhang, J. P. Chen, Biosens. Bioelectron. 2014, 62, 134.
- 41J. J. Feng, G. Zhao, J. J. Xu, H. Y. Chen, Anal. Biochem. 2005, 342, 280.