Metallophthalocyanine-Based Conjugated Microporous Polymers as Highly Efficient Photosensitizers for Singlet Oxygen Generation†
Dr. Xuesong Ding
CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bao-Hang Han
CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190 (China)
CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190 (China)Search for more papers by this authorDr. Xuesong Ding
CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bao-Hang Han
CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190 (China)
CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190 (China)Search for more papers by this authorFinancial support from the National Natural Science Foundation of China (grants no. 21304022 and 21374024) and the Ministry of Science and Technology of China (grant no. 2014CB932200) is acknowledged. We gratefully appreciate the insightful discussions and experimental support from Prof. Dr. Donglin Jiang (Institute for Molecular Science (Japan)).
Graphical Abstract
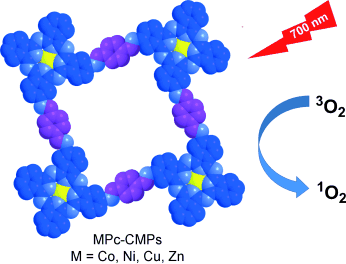
In the red: The extended π-conjugation systems of metallophthalocyanine-based conjugated microporous polymers (MPc-CMPs) results in an enhanced light-harvesting capability in the far-red region. The microporous structure of the MPc-CMPs and their excellent absorption capability for long-wavelength photons, result in them (especially ZnPc-CMP and CuPc-CMP) being promising photosensitizers with a high efficiency for 1O2 generation.
Abstract
Singlet oxygen (1O2) is of great interest because of its potential applications in photodynamic therapy, photooxidation of toxic molecules, and photochemical synthesis. Herein, we report novel metallophthalocyanine (MPc) based conjugated microporous polymers (MPc-CMPs) as photosensitizers for the generation of 1O2. The rigid microporous structure efficiently improves the exposure of the majority of the MPc units to oxygen. The MPc-CMPs also exhibit an enhanced light-harvesting capability in the far-red region through their extended π-conjugation systems. Their microporous structure and excellent absorption capability for long-wavelength photons result in the MPc-CMPs showing high efficiency for 1O2 generation upon irradiation with 700 nm light, as evident by using 1,3-diphenylisobenzofuran as an 1O2 trap. These results indicate that MPc-CMPs can be considered as promising photosensitizers for the generation of 1O2.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501732_sm_miscellaneous_information.pdf1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. R. Ogilby, Chem. Soc. Rev. 2010, 39, 3181–3209;
- 1bK. Apel, H. Hirt, Annu. Rev. Plant Biol. 2004, 55, 373–399;
- 1cA. Greer, Acc. Chem. Res. 2006, 39, 797–804;
- 1dW. Droge, Physiol. Rev. 2002, 82, 47–95;
- 1eD. E. Dolmans, D. Fukumura, R. K. Jain, Nat. Rev. Cancer 2003, 3, 380–387;
- 1fB. C. Wilson, M. S. Patterson, Phys. Med. Biol. 2008, 53, R 61–R109.
- 2
- 2aC. Schweitzer, R. Schmidt, Chem. Rev. 2003, 103, 1685–1758;
- 2bM. C. DeRosa, R. J. Crutchley, Coord. Chem. Rev. 2002, 233–234, 351–371;
- 2cO. Legrini, E. Oliveros, A. M. Braun, Chem. Rev. 1993, 93, 671–698;
- 2dA. P. Castano, P. Mroz, M. R. Hamblin, Nat. Rev. Cancer 2006, 6, 535–545;
- 2eP. Esser, B. Pohlmann, H. D. Scharf, Angew. Chem. Int. Ed. Engl. 1994, 33, 2009–2023; Angew. Chem. 1994, 106, 2093–2108;
- 2fK. Lu, C. He, W. Lin, J. Am. Chem. Soc. 2014, 136, 16712–16715.
- 3aJ. Usuda, H. Kato, T. Okunaka, K. Furukawa, H. Tsutsui, K. Yamada, Y. Suga, H. Honda, Y. Nagatsuka, T. Ohira, M. Tsuboi, T. Hirano, J. Thorac. Oncol. 2006, 1, 489–493;
- 3bS. P. Karim, R. A. Adelman, Clin. Ophthalmol. 2013, 7, 1867–1875;
- 3cM. H. Teiten, L. Bezdetnaya, P. Morliere, R. Santus, F. Guillemin, Br. J. Cancer 2003, 88, 146–152;
- 3dR. A. Lustig, T. J. Vogl, D. Fromm, R. Cuenca, R. A. Hsi, A. K. D’Cruz, Z. Krajina, M. Turic, A. Singhal, J. C. Chen, Cancer 2003, 98, 1767–1771.
- 4
- 4aK. Ishii, Coord. Chem. Rev. 2012, 256, 1556–1568;
- 4bD. C. Hone, P. I. Walker, R. Evans-Gowing, S. FitzGerald, A. Beeby, I. Chambrier, M. J. Cook, D. A. Russell, Langmuir 2002, 18, 2985–2987;
- 4cH. Shinohara, O. Tsaryova, G. Schnurpfeil, D. Wöhrle, J. Photochem. Photobiol. A 2006, 184, 50–57;
- 4dJ. Ma, J. Y. Chen, M. Idowu, T. Nyokong, J. Phys. Chem. B 2008, 112, 4465–4469.
- 5
- 5aD. Wu, F. Xu, B. Sun, R. Fu, H. He, K. Matyjaszewski, Chem. Rev. 2012, 112, 3959–4015;
- 5bX. Feng, X. Ding, D. Jiang, Chem. Soc. Rev. 2012, 41, 6010–6022;
- 5cS. Ding, W. Wang, Chem. Soc. Rev. 2013, 42, 548–568;
- 5dS. Xu, Y. Luo, B. Tan, Macromol. Rapid Commun. 2013, 34, 471–484;
- 5eN. B. McKeown, P. M. Budd, Chem. Soc. Rev. 2006, 35, 675–683.
- 6
- 6aY. Xu, S. Jin, H. Xu, A. Nagai, D. Jiang, Chem. Soc. Rev. 2013, 42, 8012–8031;
- 6bA. I. Cooper, Adv. Mater. 2009, 21, 1291–1295;
- 6cJ. Jiang, F. Su, A. Trewin, C. Wood, N. Campbell, H. Niu, C. Dickinson, A. Ganin, M. Rosseinsky, Y. Khimyak, A. Cooper, Angew. Chem. Int. Ed. 2007, 46, 8574–8578; Angew. Chem. 2007, 119, 8728–8732;
- 6dX. Zhuang, F. Zhang, D. Wu, N. Forler, H. Liang, M. Wagner, D. Gehrig, M. R. Hansen, F. Laquai, X. Feng, Angew. Chem. Int. Ed. 2013, 52, 9668–9672; Angew. Chem. 2013, 125, 9850–9854.
- 7
- 7aR. Dawson, A. Laybourn, R. Clowes, Y. Z. Khimyak, D. J. Adams, A. I. Cooper, Macromolecules 2009, 42, 8809–8816;
- 7bA. Li, R. Lu, Y. Wang, X. Wang, K. Han, W. Deng, Angew. Chem. Int. Ed. 2010, 49, 3330–3333; Angew. Chem. 2010, 122, 3402–3405;
- 7cX. Zhu, C. Tian, S. M. Mahurin, S. Chai, C. M. Wang, S. Brown, G. M. Veith, H. Luo, H. Liu, S. Dai, J. Am. Chem. Soc. 2012, 134, 10478–10484;
- 7dY. Xie, T. Wang, X. Liu, K. Zou, W. Deng, Nat. Commun. 2013, 4, 1960;
- 7eJ. Schmidt, M. Werner, A. Thomas, Macromolecules 2009, 42, 4426–4429;
- 7fQ. Chen, M. Luo, P. Hammershøj, D. Zhou, Y. Han, B. W. Laursen, C.-G. Yan, B.-H. Han, J. Am. Chem. Soc. 2012, 134, 6084–6087.
- 8
- 8aX. Liu, Y. Xu, Z. Guo, A. Nagai, D. Jiang, Chem. Commun. 2013, 49, 3233–3235;
- 8bA. Li, H. Sun, D. Tan, W. Fan, S. Wen, X. Qing, G. Li, S. Li, W. Deng, Energy Environ. Sci. 2011, 4, 2062–2065;
- 8cX. Wang, J. Liu, J. M. Bonefont, D. Yuan, P. K. Thallapally, S. Ma, Chem. Commun. 2013, 49, 1533–1535.
- 9
- 9aL. Chen, Y. Yang, D. Jiang, J. Am. Chem. Soc. 2010, 132, 9138–9143;
- 9bL. Chen, Y. Yang, Z. Guo, D. Jiang, Adv. Mater. 2011, 23, 3149–3154;
- 9cJ. Jiang, C. Wang, A. Laybourn, T. Hasell, R. Clowes, Y. Z. Khimyak, J. Xiao, S. J. Higgins, D. J. Adams, A. I. Cooper, Angew. Chem. Int. Ed. 2011, 50, 1072–1075; Angew. Chem. 2011, 123, 1104–1107;
- 9dR. K. Totten, Y. Kim, M. H. Weston, O. K. Farha, J. T. Hupp, S. T. Nguyen, J. Am. Chem. Soc. 2013, 135, 11720–11723;
- 9eK. Zhang, D. Kopetzki, P. H. Seeberger, M. Antonietti, F. Vilela, Angew. Chem. Int. Ed. 2013, 52, 1432–1436; Angew. Chem. 2013, 125, 1472–1476;
- 9fN. Huang, Y. Xu, D. Jiang, Sci. Rep. 2014, 4, 7228.
- 10
- 10aY. Xu, L. Chen, Z. Guo, A. Nagai, D. Jiang, J. Am. Chem. Soc. 2011, 133, 17622–17625;
- 10bD. Xiao, Y. Li, L. Liu, B. Wen, Z. Gu, C. Zhang, Y. Zhao, Chem. Commun. 2012, 48, 9519–9521.
- 11
- 11aY. Xu, A. Nagai, D. Jiang, Chem. Commun. 2013, 49, 1591–1593;
- 11bC. Gu, Y. Chen, Z. Zhang, S. Xue, S. Sun, K. Zhang, C. Zhong, H. Zhang, Y. Pan, Y. Lv, Y. Yang, F. Li, S. Zhang, F. Huang, Y. Ma, Adv. Mater. 2013, 25, 3443–3448;
- 11cL. Chen, Y. Honsho, S. Seki, D. Jiang, J. Am. Chem. Soc. 2010, 132, 6742–6748.
- 12
- 12aX. Liu, Y. Xu, D. Jiang, J. Am. Chem. Soc. 2012, 134, 8738–8741;
- 12bK. Wu, J. Guo, C. Wang, Chem. Commun. 2014, 50, 695–697;
- 12cC. Gu, N. Huang, J. Gao, F. Xu, Y. Xu, D. Jiang, Angew. Chem. Int. Ed. 2014, 53, 4850–4855; Angew. Chem. 2014, 126, 4950–4955.
- 13
- 13aY. Kou, Y. Xu, Z. Guo, D. Jiang, Angew. Chem. Int. Ed. 2011, 50, 8753–8757; Angew. Chem. 2011, 123, 8912–8916;
- 13bF. Vilela, K. Zhang, M. Antonietti, Energy Environ. Sci. 2012, 5, 7819–7832;
- 13cF. Xu, X. Chen, Z. Tang, D. Wu, R. Fu, D. Jiang, Chem. Commun. 2014, 50, 4788–4790;
- 13dH. Bildirir, J. Paraknowitsch, A. Thomas, Chem. Eur. J. 2014, 20, 9543–9548.
- 14
- 14aM. G. Schwab, B. Fassbender, H. W. Spiess, A. Thomas, X. Feng, K. Müllen, J. Am. Chem. Soc. 2009, 131, 7216–7217;
- 14bF. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O’Keeffe, O. M. Yaghi, J. Am. Chem. Soc. 2009, 131, 4570–4571;
- 14cF. J. Uribe-Romo, C. J. Doonan, H. Furukawa, K. Oisaki, O. M. Yaghi, J. Am. Chem. Soc. 2011, 133, 11478–11481.
- 15H. Yu, C. Shen, M. Tian, J. Qu, Z. Wang, Macromolecules 2012, 45, 5140–5150.
- 16
- 16aE. L. Spitler, W. R. Dichtel, Nat. Chem. 2010, 2, 672–677;
- 16bX. Ding, J. Guo, X. Feng, Y. Honsho, J. Guo, S. Seki, P. Maitarad, A. Saeki, S. Nagase, D. Jiang, Angew. Chem. Int. Ed. 2011, 50, 1289–1293; Angew. Chem. 2011, 123, 1325–1329;
- 16cX. Ding, L. Chen, Y. Honsho, X. Feng, O. Saengsawang, J. Guo, A. Saeki, S. Seki, S. Irle, S. Nagase, V. Parasuk, D. Jiang, J. Am. Chem. Soc. 2011, 133, 14510–14513;
- 16dE. L. Spitler, J. W. Colson, F. J. Uribe-Romo, A. R. Woll, M. R. Giovino, A. Saldivar, W. R. Dichtel, Angew. Chem. Int. Ed. 2012, 51, 2623–2627; Angew. Chem. 2012, 124, 2677–2681;
- 16eX. Ding, X. Feng, A. Saeki, S. Seki, A. Nagai, D. Jiang, Chem. Commun. 2012, 48, 8952–8954;
- 16fN. B. McKeown, S. Makhseed, P. M. Budd, Chem. Commun. 2002, 2780–2781;
- 16gA. V. Maffei, P. M. Budd, N. B. McKeown, Langmuir 2006, 22, 4225–4229;
- 16hS. Makhseed, F. Al-Kharafi, J. Samuel, B. Ateya, Catal. Commun. 2009, 10, 1284–1287.
- 17
- 17aA. Nagai, X. Chen, X. Feng, X. Ding, Z. Guo, D. Jiang, Angew. Chem. Int. Ed. 2013, 52, 3770–3774; Angew. Chem. 2013, 125, 3858–3862;
- 17bJ. Park, D. Feng, S. Yuan, H. Zhou, Angew. Chem. Int. Ed. 2015, 54, 430–435; Angew. Chem. 2015, 127, 440–445.
- 18J. R. Darwent, P. Douglas, A. Harriman, G. Porter, M.-C. Richoux, Coord. Chem. Rev. 1982, 44, 83–126.