Selective Formation of a Trisubstituted Alkene Motif by trans-Hydrostannation/Stille Coupling: Application to the Total Synthesis and Late-Stage Modification of 5,6-Dihydrocineromycin B†
M. Sc. Stephan M. Rummelt
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
These authors contributed equally to this work.
Search for more papers by this authorM. Sc. Johannes Preindl
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
These authors contributed equally to this work.
Search for more papers by this authorM. Sc. Heiko Sommer
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Alois Fürstner
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)Search for more papers by this authorM. Sc. Stephan M. Rummelt
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
These authors contributed equally to this work.
Search for more papers by this authorM. Sc. Johannes Preindl
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
These authors contributed equally to this work.
Search for more papers by this authorM. Sc. Heiko Sommer
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Alois Fürstner
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)Search for more papers by this authorGenerous financial support by the MPG is gratefully acknowledged. We thank J. Rust and Prof. C. W. Lehmann for solving the X-ray structure, the Analytical Departments of our Institute for excellent support, Dr. J. Herrmann and Prof. R. Müller, Helmholtz Institute for Pharmaceutical Research Saarland, Saarbrücken, for the biological data, and Umicore AG & Ko KG, Hanau, for a generous gift of noble metal salts.
Graphical Abstract
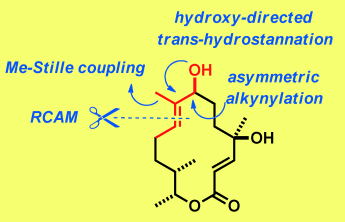
Directed although indirect: The antibiotic dihydrocineromyin B is one of countless natural products featuring an E-configured 2-methylbut-2-en-1-ol substructure (see picture, RCAM=ring-closing alkyne metathesis). The key step in the synthesis was a ruthenium-catalyzed hydroxy-directed trans-hydrostannation of an alkyne. The method is efficient, broadly applicable, and suited to late-stage diversification.
Abstract
Countless natural products of polyketide origin have an E-configured 2-methyl-but-2-en-1-ol substructure. An unconventional entry into this important motif was developed as part of a concise total synthesis of 5,6-dihydrocineromycin B. The choice of this particular target was inspired by a recent study, which suggested that the cineromycin family of antibiotics might have overlooked lead qualities, although our biodata do not necessarily support this view. The new approach consists of a sequence of alkyne metathesis followed by a hydroxy-directed trans-hydrostannation and a largely unprecedented methyl-Stille coupling. The excellent yield and remarkable selectivity with which the signature trisubstituted alkene site of the target was procured is noteworthy considering the rather poor outcome of a classical ring-closing metathesis reaction. Moreover, the unorthodox ruthenium-catalyzed trans-hydrostannation is shown to be a versatile handle for diversity-oriented synthesis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501608_sm_miscellaneous_information.pdf4.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. Nagahama, M. Suzuki, S. Awataguchi, T. Okuda, J. Antibiot. Ser. A 1967, 20, 261–266;
- 1bT. Furumai, N. Nagahama, T. Okuda, J. Antibiot. 1968, 21, 85–90;
- 1cL. Slechta, J. Cialdella, H. Hoeksema, J. Antibiot. 1978, 31, 319–323;
- 1dK. Harada, F. Nishida, H. Tagaki, M. Suzuki, T. Iwashita, J. Antibiot. 1984, 37, 1187–1196;
- 1eA. Taddei, A. Zeeck, J. Antibiot. 1997, 50, 526–528.
- 2
- 2aA. Furusaki, T. Matsumoto, K. Harada, M. Suzuki, K. Kinoshita, M. Hayashi, K. Nakatsu, Bull. Chem. Soc. Jpn. 1983, 56, 3042–3046;
- 2bR. C. Thomas, C. G. Chidester, J. Antibiot. 1982, 35, 1658–1664.
- 3N. Miyairi, M. Takashima, K. Shimizu, H. Sakai, J. Antibiot. Ser. A 1966, 19, 56–62.
- 4 Macrolide Antibiotics. Chemistry, Biology, and Practice (Ed.: ), 2nd ed., Academic Press, San Diego, 2002.
- 5N. Koyama, M. Yotsumoto, H. Onaka, H. Tomoda, J. Antibiot. 2013, 66, 303–304.
- 6For a pertinent review, see F. von Nussbaum, M. Brands, B. Hinzen, S. Weigand, D. Häbich, Angew. Chem. Int. Ed. 2006, 45, 5072–5129; Angew. Chem. 2006, 118, 5194–5254.
- 7Moreover, Ref. [5] suggests that 1 interferes with the peptidoglycan biosynthesis, a mode of action that is unprecedented for 14-membered macrolides; earlier studies had proposed inhibition of the nicotinate biosynthesis, see F. Reusser, J. Bacteriol. 1969, 100, 11–13.
- 81 was also reported to be a reversible inhibitor of human prolyl endopeptidases, see C. Christner, G. Küllertz, G. Fischer, M. Zerlin, S. Gabley, R. Thiericke, A. Taddei, A. Zeeck, J. Antibiot. 1998, 51, 368–371.
- 9
- 9aK. Burkhardt, H.-P. Fiedler, S. Grabley, R. Thiericke, A. Zeeck, J. Antibiot. 1996, 49, 432–437;
- 9bA. Schneider, J. Späth, S. Breiding-Mack, A. Zeeck, S. Grabley, R. Thiericke, J. Antibiot. 1996, 49, 438–446;
- 9cH.-J. Schiewe, A. Zeeck, J. Antibiot. 1999, 52, 635–642.
- 10D. Tanner, P. Somfai, Tetrahedron 1987, 43, 4395–4406.
- 11T. Takahashi, H. Watanabe, T. Kitahara, Tetrahedron Lett. 2003, 44, 9219–9222.
- 12L. F. Tietze, L. Völkel, Angew. Chem. Int. Ed. 2001, 40, 901–902; Angew. Chem. 2001, 113, 925–927.
- 13G. Li, X. Yang, H. Zhai, J. Org. Chem. 2009, 74, 1356–1359.
- 14G. V. Reddy, R. S. C. Kumar, B. Siva, K. S. Babu, J. M. Rao, Synlett 2012, 2677–2681.
- 15
- 15aR. M. Wilson, S. J. Danishefsky, Angew. Chem. Int. Ed. 2010, 49, 6032–6056; Angew. Chem. 2010, 122, 6168–6193;
- 15bA. M. Szpilman, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 9592–9628; Angew. Chem. 2010, 122, 9786–9823;
- 15cA. Fürstner, Isr. J. Chem. 2011, 51, 329–345;
- 15dJ.-Y. Wach, K. Gademann, Synlett 2012, 163–170.
- 16Although the RCM product was the correct isomer, it is emphasized that RCM is substrate-controlled; catalysts able to enforce the formation of E-configured trisubstituted olefins are currently unknown. For general discussions, see
- 16aA. Fürstner, Science 2013, 341, 1357 (UNSP 1229713);
- 16bA. H. Hoveyda, J. Org. Chem. 2014, 79, 4763–4792.
- 17The difficulties in forming trisubstituted alkenes flanked by an -OR group by RCM and the potential lack of control over the olefin geometry is by no means a specific problem of 3; for other instructive cases, see
- 17aB. M. Trost, G. Dong, J. A. Vance, Chem. Eur. J. 2010, 16, 6265–6277;
- 17bI. Ramírez-Fernández, I. G. Collado, R. Hernández-Galán, Synlett 2008, 339–342.
- 18A. Fürstner, Angew. Chem. Int. Ed. 2013, 52, 2794–2819; Angew. Chem. 2013, 125, 2860–2887.
- 19
- 19aA. Fürstner, G. Seidel, Angew. Chem. Int. Ed. 1998, 37, 1734–1736;
10.1002/(SICI)1521-3773(19980703)37:12<1734::AID-ANIE1734>3.0.CO;2-6 CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 1758–1760;10.1002/(SICI)1521-3757(19980619)110:12<1758::AID-ANGE1758>3.0.CO;2-I Web of Science® Google Scholar
- 19bA. Fürstner, O. Guth, A. Rumbo, G. Seidel, J. Am. Chem. Soc. 1999, 121, 11108–11113.
- 20S. M. Rummelt, A. Fürstner, Angew. Chem. Int. Ed. 2014, 53, 3626–3630; Angew. Chem. 2014, 126, 3700–3704.
- 21See also:
- 21aB. Sundararaju, A. Fürstner, Angew. Chem. Int. Ed. 2013, 52, 14050–14054; Angew. Chem. 2013, 125, 14300–14304;
- 21bK. Radkowski, B. Sundararaju, A. Fürstner, Angew. Chem. Int. Ed. 2013, 52, 355–360; Angew. Chem. 2013, 125, 373–378;
- 21cA. Fürstner, Angew. Chem. Int. Ed. 2014, 53, 8587–8598; Angew. Chem. 2014, 126, 8728–8740.
- 22V. Farina, V. Krishnamurty, W. J. Scott, Org. React. 1997, 50, 1–652.
- 23P. A. Bartlett, D. P. Richardson, J. Myerson, Tetrahedron 1984, 40, 2317–2327.
- 24A. G. M. Barrett, R. A. E. Carr, S. V. Attwood, G. Richardson, N. D. A. Walshe, J. Org. Chem. 1986, 51, 4840–4856.
- 25
- 25aK. Lehr, S. Schulthoff, Y. Ueda, R. Mariz, L. Leseurre, B. Gabor, A. Fürstner, Chem. Eur. J. 2015, 21, 219–227;
- 25bK. Lehr, R. Mariz, L. Leseurre, B. Gabor, A. Fürstner, Angew. Chem. Int. Ed. 2011, 50, 11373–11377; Angew. Chem. 2011, 123, 11575–11579.
- 26
- 26aM. Fuchs, A. Fürstner, Angew. Chem. Int. Ed. 2015, 54, 3978–3982; Angew. Chem. 2015, 127, 4050–4054;
- 26bL. Hoffmeister, P. Persich, A. Fürstner, Chem. Eur. J. 2014, 20, 4396–4402;
- 26cK. Lehr, A. Fürstner, Tetrahedron 2012, 68, 7695–7700.
- 27D. P. Canterbury, G. C. Micalizio, Org. Lett. 2011, 13, 2384–2387.
- 28
- 28aD. Boyall, D. E. Frantz, E. M. Carreira, Org. Lett. 2002, 4, 2605–2606;
- 28bD. E. Frantz, R. Fässler, E. M. Carreira, J. Am. Chem. Soc. 2000, 122, 1806–1807.
- 29For a pertinent example, see J. Wu, J. S. Panek, Angew. Chem. Int. Ed. 2010, 49, 6165–6168; Angew. Chem. 2010, 122, 6301–6304.
- 30D. J. Schauer, P. Helquist, Synthesis 2006, 3654–3660.
- 31
- 31aJ. Heppekausen, R. Stade, A. Kondoh, G. Seidel, R. Goddard, A. Fürstner, Chem. Eur. J. 2012, 18, 10281–10299;
- 31bJ. Heppekausen, R. Stade, R. Goddard, A. Fürstner, J. Am. Chem. Soc. 2010, 132, 11045–11057;
- 31cP. Persich, J. Llaveria, R. Lhermet, T. de Haro, R. Stade, A. Kondoh, A. Fürstner, Chem. Eur. J. 2013, 19, 13047–13058.
- 32For selected applications of this particular catalyst to natural product synthesis, see
- 32aV. Hickmann, A. Kondoh, B. Gabor, M. Alcarazo, A. Fürstner, J. Am. Chem. Soc. 2011, 133, 13471–13480;
- 32bK. Micoine, A. Fürstner, J. Am. Chem. Soc. 2010, 132, 14064–14066;
- 32cW. Chaładaj, M. Corbet, A. Fürstner, Angew. Chem. Int. Ed. 2012, 51, 6929–6933; Angew. Chem. 2012, 124, 7035–7039;
- 32dS. Benson, M.-P. Collin, A. Arlt, B. Gabor, R. Goddard, A. Fürstner, Angew. Chem. Int. Ed. 2011, 50, 8739–8744; Angew. Chem. 2011, 123, 8898–8903;
- 32eL. Brewitz, J. Llaveria, A. Yada, A. Fürstner, Chem. Eur. J. 2013, 19, 4532–4537.
- 33T. D. Tilley, R. H. Grubbs, J. E. Bercaw, Organometallics 1984, 3, 274–278.
- 34An in-depth discussion will be the subject of a forthcoming publication from our group.
- 35We are aware of one study reporting the Stille coupling of 11CH3I; in this case, however, the stannane was used in huge excess, see T. Hosoya, K. Sumi, H. Doi, M. Wakao, M. Suzuki, Org. Biomol. Chem. 2006, 4, 410–415.
- 36In contrast, Stille-type reactions with higher alkyl halides are more common; for leading references see the following and literature cited therein:
- 36aH. Tang, K. Menzel, G. C. Fu, Angew. Chem. Int. Ed. 2003, 42, 5079–5082; Angew. Chem. 2003, 115, 5233–5236;
- 36bK. Menzel, G. C. Fu, J. Am. Chem. Soc. 2003, 125, 3718–3719;
- 36cD. A. Powell, T. Maki, G. C. Fu, J. Am. Chem. Soc. 2005, 127, 510–511.
- 37E. J. Corey, J. A. Katzenellenbogen, G. H. Posner, J. Am. Chem. Soc. 1967, 89, 4245–4247.
- 38
- 38aP. Dimopoulos, A. Athlan, S. Manaviazar, K. J. Hale, Org. Lett. 2005, 7, 5373–5376;
- 38bK. J. Hale, M. Grabski, S. Manaviazar, M. Maczka, Org. Lett. 2014, 16, 1164–1167;
- 38cK. J. Hale, M. Maczka, A. Kaur, S. Manaviazar, M. Ostovar, M. Grabski, Org. Lett. 2014, 16, 1168–1171;
- 38dN. A. Morra, B. L. Pagenkopf, Org. Lett. 2011, 13, 572–575;
- 38eY. Iwasaki, R. Matsui, T. Suzuki, A. Nakazaki, S. Kobayashi, Chem. Pharm. Bull. 2011, 59, 522–524.
- 39
- 39aE. J. Corey, R. H. Wollenberg, J. Am. Chem. Soc. 1974, 96, 5581–5583;
- 39bE. Piers, J. S. M. Wai, Can. J. Chem. 1994, 72, 146–157;
- 39cT. N. Mitchell, W. Reimann, J. Organomet. Chem. 1985, 281, 163–171;
- 39dM. Bénéchie, T. Skrydstrup, F. Khuong-Huu, Tetrahedron Lett. 1991, 32, 7535–7538.
- 40A. Fürstner, J.-A. Funel, M. Tremblay, L. C. Bouchez, C. Nevado, M. Waser, J. Ackerstaff, C. C. Stimson, Chem. Commun. 2008, 2873–2875.
- 41For applications, see
- 41aA. Fürstner, J. Ackerstaff, Chem. Commun. 2008, 2870–2872;
- 41bA. Larivée, J. B. Unger, M. Thomas, C. Wirtz, C. Dubost, S. Handa, A. Fürstner, Angew. Chem. Int. Ed. 2011, 50, 304–309; Angew. Chem. 2011, 123, 318–323;
- 41cA. Fürstner, L. C. Bouchez, L. Morency, J.-A. Funel, V. Liepins, F.-H. Porée, R. Gilmour, D. Laurich, F. Beaufils, M. Tamiya, Chem. Eur. J. 2009, 15, 3983–4010;
- 41dJ. Gagnepain, E. Moulin, A. Fürstner, Chem. Eur. J. 2011, 17, 6964–6972;
- 41eD. Mailhol, J. Willwacher, N. Kausch-Busies, E. E. Rubitski, Z. Sobol, M. Schuler, M.-H. Lam, S. Musto, F. Loganzo, A. Maderna, A. Fürstner, J. Am. Chem. Soc. 2014, 136, 15719–15729.
- 42For selected applications by other groups, see
- 42aA. Francais, A. Leyva, G. Etxebarria-Jardi, S. V. Ley, Org. Lett. 2010, 12, 340–343;
- 42bI. Paterson, S. B. J. Kan, L. J. Gibson, Org. Lett. 2010, 12, 3724–3727;
- 42cL. Otero, B. Vaz, R. Álvarez, Á. R. de Lera, Chem. Commun. 2013, 49, 5043–5045;
- 42dS. Müller, T. Mayer, F. Sasse, M. E. Maier, Org. Lett. 2011, 13, 3940–3943;
- 42eH. Renata, Q. Zhou, P. S. Baran, Science 2013, 339, 59–63;
- 42fH. Miyatake-Ondozabal, E. Kaufmann, K. Gademann, Angew. Chem. Int. Ed. 2015, 54, 1933–1936; Angew. Chem. 2015, 127, 1953–1956.
- 43G. D. Allred, L. S. Liebeskind, J. Am. Chem. Soc. 1996, 118, 2748–2749.
- 44J. Srogl, G. D. Allred, L. S. Liebeskind, J. Am. Chem. Soc. 1997, 119, 12376–12377.
- 45Except for the optical rotation. The large difference between the [α]D value of analytically pure synthetic 3 and that of the natural sample has already been noted before, see Ref. [13].
- 46A. C. Regan in Comprehensive Functional Group Transformations, Vol. 1 (Eds.: ), Elsevier Science, Oxford, 1995, pp. 501–551.
- 47
- 47aT. Takeda, F. Kanamori, H. Matsusita, T. Fujiwara, Tetrahedron Lett. 1991, 32, 6563–6566;
- 47bD. Madec, J.-P. Férézou, Eur. J. Org. Chem. 2006, 92–104.
- 48CCDC 1048778 (20) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 49In a liquid culture dilution assay, the minimum inhibition concentration (MIC) of 3 against S. aureus Newman was 16 μg mL−1, whereas the MIC of 3, 15, 17, 18, 19, and 20 was invariably ≥64 μg mL−1 against all other tested bacterial and fungal strains.