In Situ Generation of Difluoromethyl Diazomethane for [3+2] Cycloadditions with Alkynes†
Corresponding Author
Dr. Pavel K. Mykhailiuk
Enamine Ltd. Matrosova 23, 01103 Kyiv (Ukraine) http://www.enamine.net
Taras Shevchenko National University of Kyiv, Chemistry Department, Volodymyrska 64, 01601 Kyiv (Ukraine)
Enamine Ltd. Matrosova 23, 01103 Kyiv (Ukraine) http://www.enamine.netSearch for more papers by this authorCorresponding Author
Dr. Pavel K. Mykhailiuk
Enamine Ltd. Matrosova 23, 01103 Kyiv (Ukraine) http://www.enamine.net
Taras Shevchenko National University of Kyiv, Chemistry Department, Volodymyrska 64, 01601 Kyiv (Ukraine)
Enamine Ltd. Matrosova 23, 01103 Kyiv (Ukraine) http://www.enamine.netSearch for more papers by this authorAll chemicals were provided by Enamine Ltd. I am grateful to Dr. S. Shishkina for X-ray studies, to Prof. T. Brigaud for insightful comments on the reaction mechanism, to R. Iminov, B. Chalyk, V. Arkhipov, and O. Mashkov for their help with managing this work, and to C. Thinnes and Dr. V. Kubyshkin for proofreading the manuscript.
Graphical Abstract
Abstract
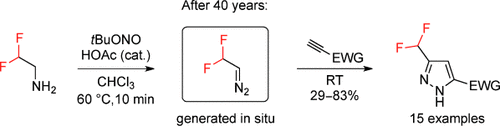
A novel approach to agrochemically important difluoromethyl-substituted pyrazoles has been developed based on the elusive reagent CF2HCHN2, which was synthesized (generated in situ) for the first time and employed in [3+2] cycloaddition reactions with alkynes. The reaction is extremely practical as it is a one-pot process, does not require a catalyst or the isolation of the potentially toxic and explosive gaseous intermediate, and proceeds in a common solvent, namely chloroform, in air. The reaction is also scalable and allows for the preparation of the target pyrazoles on gram scale.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501529_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Bioorganic and Medicinal Chemistry of Fluorine (Eds.: ), Wiley, Hoboken, 2008;
- 1b Fluorine in Medicinal Chemistry and Chemical Biology (Ed.: ), Blackwell Publishing, Oxford, 2009;
10.1002/9781444312096 Google Scholar
- 1c Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications (Eds.: ), Imperial College Press, London, 2012.
- 2
- 2aH.-J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander, M. Stahl, ChemBioChem 2004, 5, 637;
- 2bC. Isanbor, D. O’Hagan, J. Fluorine Chem. 2006, 127, 303;
- 2cK. L. Kirk, Org. Process Res. Dev. 2008, 12, 305;
- 2dS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320;
- 2eW. K. Hagmann, J. Med. Chem. 2008, 51, 4359.
- 3More than 50 FDA-approved drugs contain a CF3 group and 5 the CHF2 group; see: D. S. Wishart, C. Knox, A. C. Guo, D. Cheng, S. Shrivastaya, D. Tzur, B. Gautam, M. Hassanali, Nucleic Acids Res. 2008, 36 (Database issue), D. 901.
- 4
- 4aJ.-A. Ma, D. Cahard, Chem. Rev. 2004, 104, 6119;
- 4bY. Macé, E. Magnier, Eur. J. Org. Chem. 2012, 2479;
- 4cC. Ni, M. Hu, J. Hu, Chem. Rev. 2015, 115, 765;
- 4dO. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475;
- 4eJ. Charpentier, N. Früh, A. Togni, Chem. Rev. 2015, 115, 650;
- 4fS. Barata-Vallejo, B. Lantano, A. Postigo, Chem. Eur. J. 2014, 20, 16806;
- 4gS. Roy, B. T. Gregg, G. W. Gribble, V.-D. Le, S. Roy, Tetrahedron 2011, 67, 2161;
- 4hJ. Nie, H.-C. Guo, D. Cahard, J.-A. Ma, Chem. Rev. 2011, 111, 455.
- 5H. Gilman, R. G. Jones, J. Am. Chem. Soc. 1943, 65, 1458.
- 6For reports by Carreira and co-workers on the use of CF3CHN2, see:
- 6aB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 938; Angew. Chem. 2010, 122, 950;
- 6bB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 4294; Angew. Chem. 2010, 122, 4390;
- 6cB. Morandi, B. Mariampillai, E. M. Carreira, Angew. Chem. Int. Ed. 2011, 50, 1101; Angew. Chem. 2011, 123, 1133;
- 6dB. Morandi, J. Cheang, E. M. Carreira, Org. Lett. 2011, 13, 3080;
- 6eB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2011, 50, 9085; Angew. Chem. 2011, 123, 9251;
- 6fB. Morandi, E. M. Carreira, Org. Lett. 2011, 13, 5984;
- 6gS. A. Künzi, B. Morandi, E. M. Carreira, Org. Lett. 2012, 14, 1900;
- 6hJ. Y. Hamilton, B. Morandi, E. M. Carreira, Synthesis 2013, 1857.
- 7For contributions from Ma et al. on the use of CF3CHN2, see:
- 7aC.-B. Liu, W. Meng, F. Li, S. Wang, J. Nie, J.-A. Ma, Angew. Chem. Int. Ed. 2012, 51, 6227; Angew. Chem. 2012, 124, 6331;
- 7bF. Li, J. Nie, L. Sun, Y. Zheng, J.-A. Ma, Angew. Chem. Int. Ed. 2013, 52, 6255; Angew. Chem. 2013, 125, 6375;
- 7cH.-Y. Xiong, Z.-Y. Yang, Z. Chen, J.-L. Zeng, J. Nie, J.-A. Ma, Chem. Eur. J. 2014, 20, 8325;
- 7dF.-G. Zhang, Y. Wei, Y.-P. Yi, J. Nie, J.-A. Ma, Org. Lett. 2014, 16, 3122;
- 7eS. Wang, J. Nie, Y. Zheng, J.-A. Ma, Org. Lett. 2014, 16, 1606;
- 7fL. Sun, J. Nie, Y. Zheng, J.-A. Ma, J. Fluorine Chem. 2015, DOI: .
- 8For contributions of Molander and co-workers on the use of CF3CHN2, see:
- 8aO. A. Argintaru, D. Ryu, I. Aron, G. A. Molander, Angew. Chem. Int. Ed. 2013, 52, 13656; Angew. Chem. 2013, 125, 13901;
- 8bG. A. Molander, L. Cavalcanti, Org. Lett. 2013, 15, 3166;
- 8cG. A. Molander, D. Ryu, Angew. Chem. Int. Ed. 2014, 53, 14181; Angew. Chem. 2014, 126, 14405.
- 9For our contributions on this topic, see:
- 9aP. K. Mykhailiuk, S. Afonin, G. V. Palamarchuk, O. V. Shishkin, A. S. Ulrich, I. V. Komarov, Angew. Chem. Int. Ed. 2008, 47, 5765; Angew. Chem. 2008, 120, 5849;
- 9bP. K. Mykhailiuk, S. Afonin, A. S. Ulrich, I. V. Komarov, Synthesis 2008, 1757;
- 9cO. S. Artamonov, P. K. Mykhailiuk, N. M. Voievoda, D. M. Volochnyuk, I. V. Komarov, Synthesis 2010, 443;
- 9dO. S. Artamonov, E. Y. Slobodyanyuk, O. V. Shishkin, I. V. Komarov, P. K. Mykhailiuk, Synthesis 2013, 225;
- 9eO. S. Artamonov, E. Y. Slobodyanyuk, D. M. Volochnyuk, I. V. Komarov, A. A. Tolmachev, P. K. Mykhailiuk, Eur. J. Org. Chem. 2014, 3592;
- 9fE. Y. Slobodyanyuk, O. S. Artamonov, O. V. Shishkin, P. K. Mykhailiuk, Eur. J. Org. Chem. 2014, 2487.
- 10For recent contributions of other groups on the use of CF3CHN2, see:
- 10aP. Le Maux, S. Juillard, G. Simonneaux, Synthesis 2006, 1701;
- 10bM. A. J. Duncton, L. Ayala, C. Kaub, S. Janagani, W. T. Edwards, N. Orike, K. Ramamoorthy, J. Kincaid, G. G. Kelly, Tetrahedron Lett. 2010, 51, 1009;
- 10cI. Suárez del Villar, A. Gradillas, J. Pérez-Castells, Eur. J. Org. Chem. 2010, 5850;
- 10dM. A. J. Duncton, R. Singh, Org. Lett. 2013, 15, 4284;
- 10eZ. Chai, J.-P. Bouillon, D. Cahard, Chem. Commun. 2012, 48, 9471;
- 10fG. Wu, Y. Deng, C. Wu, X. Wang, Y. Zhang, J. Wang, Eur. J. Org. Chem. 2014, 4477;
- 10gT.-R. Li, S.-W. Duan, W. Ding, Y. Y. Liu, J. R. Chen, L.-Q. Lu, W.-J. Xiao, J. Org. Chem. 2014, 79, 2296.
- 11J. H. Atherton, R. Fields, R. N. Haszeldine, J. Chem. Soc. C 1971, 366.
- 12P. K. Mykhailiuk, Org. Biomol. Chem. 2015, 13, 3438.
- 13 Classics in Total Synthesis III (Eds.: ), Wiley, Hoboken, 2011.
- 14For some popular difluoromethylation reagents, see:
- 14aY. Zhao, W. Huang, J. Zheng, J. Hu, Org. Lett. 2011, 13, 5342 (TMSCF2H);
- 14bV. V. Levin, A. L. Trifonov, A. A. Zemtsov, M. I. Struchkova, D. E. Arkhipov, A. D. Dilman, Org. Lett. 2014, 16, 6256 (Ph3P+CF2CO2−);
- 14cG. K. S. Prakash, J. Hu, Y. Wang, G. A. Olah, J. Fluorine Chem. 2005, 126, 527 (PhSCF2TMS);
- 14dG. K. S. Prakash, J. Hu, Y. Wang, G. A. Olah, Eur. J. Org. Chem. 2005, 2218 (PhSCF2H);
- 14eQ.-Y. Chen, S. Wu, J. Fluorine Chem. 1989, 44, 433 (FSO2CF2CO2H);
- 14fG. K. S. Prakash, C. Weber, S. Chacko, G. A. Olah, Org. Lett. 2007, 9, 1863 (Ar2SCHF2+BF4−);
- 14gW. Zhang, F. Wang, J. Hu, Org. Lett. 2009, 11, 2109 [PhS(O)(NTs)CHF2].
- 15For an analysis of the gaps in chemical space, see: T. Fink, J.-L. Reymond, J. Chem. Inf. Model. 2007, 47, 342.
- 16Over the past five years, difluoromethylated pyrazoles were contained in more than 200 patents of well-known pharmaceutical and agrochemical companies (Reaxys DB).
- 17For a review on difluoromethylated pyrazoles in agrochemistry, see: F. Giornal, S. Pazenok, L. Rodefeld, N. Lui, J.-P. Vors, F. R. Leroux, J. Fluorine Chem. 2013, 152, 2.
- 18Recently, C2F5CHN2 was also generated in aqueous media; see:
- 18aP. Mykhailiuk, Chem. Eur. J. 2014, 20, 4942;
- 18bS. K. Ritter, Chem. Eng. News 2014, 92, 26;
- 18cP. K. Mykhailiuk, Beilstein J. Org. Chem. 2015, 11, 16.
- 19N. Takamura, T. Mizoguchi, Tetrahedron 1975, 31, 227.
- 20G. Maas in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products (Eds.: ), Wiley, New York, 2002, pp. 539–621.
10.1002/0471221902.ch8 Google Scholar
- 21 Prudent Practices for Handling Hazardous Chemicals in Laboratories, National Academy Press, Washington, 1981, pp. 57–68.
- 22CCDC 1037925 (3 a), 1037927 (4 a), 1048720 (7 a), 1037926 (12 a), 1048719 (16 a), and 1037928 (17 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 23Q. Sha, H. Liu, Y. Wie, Eur. J. Org. Chem. 2014, 7707;
- 23bR. T. Iminov, A. V. Mashkov, I. I. Vyzir, B. A. Chalyk, A. V. Tverdokhlebov, P. K. Mykhailiuk, L. N. Babichenko, A. A. Tolmachev, Y. M. Volovenko, A. Biitseva, O. V. Shishkin, S. V. Shishkina, Eur. J. Org. Chem. 2015, 886;
- 23cF. Giornal, G. Landelle, N. Lui, J.-P. Vors, S. Pazenok, F. R. Leroux, Org. Process Res. Dev. 2014, 18, 1002;
- 23dR. Román, A. Navarro, D. Wodka, M. Alvim-Gaston, S. Husain, N. Franklin, A. Simón-Fuentes, S. Fustero, Org. Process Res. Dev. 2014, 18, 1027;
- 23eS. Pazenok, F. Giornal, G. Landelle, N. Lui, J.-P. Vors, F. R. Leroux, Eur. J. Org. Chem. 2013, 4249.