Synthesis and Characterization of a Gold Vinylidene Complex Lacking π-Conjugated Heteroatoms†
Robert J. Harris
French Family Science Center, Duke University, Durham, NC 27708-0346 (USA)
Search for more papers by this authorCorresponding Author
Prof. Ross A. Widenhoefer
French Family Science Center, Duke University, Durham, NC 27708-0346 (USA)
French Family Science Center, Duke University, Durham, NC 27708-0346 (USA)Search for more papers by this authorRobert J. Harris
French Family Science Center, Duke University, Durham, NC 27708-0346 (USA)
Search for more papers by this authorCorresponding Author
Prof. Ross A. Widenhoefer
French Family Science Center, Duke University, Durham, NC 27708-0346 (USA)
French Family Science Center, Duke University, Durham, NC 27708-0346 (USA)Search for more papers by this authorWe acknowledge the NSF (CHE-1213957) for support of this research.
Graphical Abstract
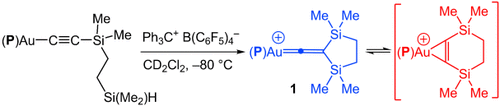
Good as gold: Cationic gold (β,β-disilyl)vinylidene complex 1 was generated by addition of a pendant silylium ion to the CC bond of a gold acetylide complex (see scheme, P=PtBu2(o-biphenyl)). The vinylidene C1 and C2 atoms of 1 undergo facile interconversion, presumably via a π-disilacyclohexyne intermediate. 29Si NMR analysis of 1 indicates delocalization of positive charge onto both the β-silyl groups and the (P)Au fragment.
Abstract
Hydride abstraction from the gold (disilyl)ethylacetylide complex [(P)Au{η1-CCSi(Me)2CH2CH2SiMe2H}] (P=P(tBu)2o-biphenyl) with triphenylcarbenium tetrakis(pentafluorophenyl)borate at −20 °C formed the cationic gold (β,β-disilyl)vinylidene complex [(P)AuCCSi(Me)2CH2CH2Si(Me)2]+B(C6F5)4− with ≥90 % selectivity. 29Si NMR analysis of this complex pointed to delocalization of positive charge onto both the β-silyl groups and the (P)Au fragment. The C1 and C2 carbon atoms of the vinylidene complex underwent facile interconversion (ΔG≠=9.7 kcal mol−1), presumably via the gold π-disilacyclohexyne intermediate [(P)Au{η2-CCSi(Me)2CH2CH2Si(Me)2}]+B(C6F5)4−.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501474_sm_miscellaneous_information.pdf640.3 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Obradors, A. M. Echavarren, Acc. Chem. Res. 2014, 47, 902–912;
- 1bA. Fürstner, Acc. Chem. Res. 2014, 47, 925–938;
- 1cP. Y. Toullec, V. Michelet, Top. Curr. Chem. 2011, 302, 31–80;
- 1dA. M. Echavarren, E. Jiménez-Núñez, Top. Catal. 2010, 53, 924–930;
- 1eA. Fürstner, Chem. Soc. Rev. 2009, 38, 3208–3221;
- 1fE. Jiménez-Núñez, A. M. Echavarren, Chem. Rev. 2008, 108, 3326–3350;
- 1gV. Michelet, P. Y. Toullec, J. P. Genêt, Angew. Chem. Int. Ed. 2008, 47, 4268–4315; Angew. Chem. 2008, 120, 4338–4386.
- 2A. Correa, N. Marion, L. Fensterbank, M. Malacria, S. P. Nolan, L. Cavallo, Angew. Chem. Int. Ed. 2008, 47, 718–721; Angew. Chem. 2008, 120, 730–733.
- 3D. Qian, J. Zhang, Chem. Soc. Rev. 2015, 44, 677–698.
- 4
- 4aL. Zhang, Acc. Chem. Res. 2014, 47, 877–888;
- 4bH.-S. Yeom, S. Shin, Acc. Chem. Res. 2014, 47, 966–977.
- 5For general reviews of gold catalysis, see
- 5aM. Bandini, Chem. Soc. Rev. 2011, 40, 1358–1367;
- 5bA. Corma, A. Leyva-Peŕez, M. J. Sabater, Chem. Rev. 2011, 111, 1657–1712;
- 5cM. Rudolph, A. S. K. Hashmi, Chem. Commun. 2011, 47, 6536–6544;
- 5dN. Krause, C. Winter, Chem. Rev. 2011, 111, 1994–2009;
- 5eT. C. Boorman, I. Larrosa, Chem. Soc. Rev. 2011, 40, 1910–1925;
- 5fA. Pradal, P. Y. Toullec, V. Michelet, Synthesis 2011, 1501–1514.
- 6
- 6aA. S. K. Hashmi, Angew. Chem. Int. Ed. 2008, 47, 6754–6756; Angew. Chem. 2008, 120, 6856–6858;
- 6bA. Fürstner, L. Morency, Angew. Chem. Int. Ed. 2008, 47, 5030–5033; Angew. Chem. 2008, 120, 5108–5111;
- 6cA. M. Echavarren, Nat. Chem. 2009, 1, 431–433;
- 6dG. Seidel, R. Mynott, A. Fürstner, Angew. Chem. Int. Ed. 2009, 48, 2510–2513; Angew. Chem. 2009, 121, 2548–2551;
- 6eD. Benitez, N. D. Shapiro, E. Tkatchouk, Y. Wang, W. A. Goddard, F. D. Toste, Nat. Chem. 2009, 1, 482–486;
- 6fG. Seidel, B. Gabor, R. Goddard, B. Heggen, W. Thiel, A. Fürstner, Angew. Chem. Int. Ed. 2014, 53, 879–882; Angew. Chem. 2014, 126, 898–901;
- 6gG. Ciancaleoni, L. Biasiolo, G. Bistoni, A. Macchioni, F. Tarantelli, D. Zuccaccia, L. Belpassi, Chem. Eur. J. 2015, 21, 2467–2473.
- 7M. W. Hussong, F. Rominger, P. Krämer, B. F. Straub, Angew. Chem. Int. Ed. 2014, 53, 9372–9375; Angew. Chem. 2014, 126, 9526–9529.
- 8G. Seidel, A. Fürstner, Angew. Chem. Int. Ed. 2014, 53, 4807–4811; Angew. Chem. 2014, 126, 4907–4911.
- 9R. E. M. Brooner, R. A. Widenhoefer, Chem. Commun. 2014, 50, 2420–2423.
- 10R. J. Harris, R. A. Widenhoefer, Angew. Chem. Int. Ed. 2014, 53, 9369–9371; Angew. Chem. 2014, 126, 9523–9525.
- 11For gas-phase studies of gold carbene complexes, see
- 11aA. Fedorov, M.-E. Moret, P. Chen, J. Am. Chem. Soc. 2008, 130, 8880–8881;
- 11bA. Fedorov, P. Chen, Organometallics 2009, 28, 1278–1281;
- 11cL. Batiste, A. Fedorov, P. Chen, Chem. Commun. 2010, 46, 3899–3901;
- 11dA. Fedorov, L. Batiste, A. Bach, D. M. Birney, P. Chen, J. Am. Chem. Soc. 2011, 133, 12162–12171;
- 11eD. H. Ringger, P. Chen, Angew. Chem. Int. Ed. 2013, 52, 4686–4689; Angew. Chem. 2013, 125, 4784–4787;
- 11fD. H. Ringger, I. J. Kobylianskii, D. Serra, P. Chen, Chem. Eur. J. 2014, 20, 14270–14281;
- 11gL. Batiste, P. Chen, J. Am. Chem. Soc. 2014, 136, 9296–9307.
- 12For additional examples of heteroatom-stabilized gold carbenes, see
- 12aL.-P. Liu, B. Xu, M. S. Mashuta, G. B. Hammond, J. Am. Chem. Soc. 2008, 130, 17642–17643;
- 12bM. N. Fañanás-Mastral, F. Aznar, Organometallics 2009, 28, 666–668;
- 12cU. Schubert, K. Ackermann, R. Aumann, Cryst. Struct. Commun. 1982, 11, 591–594;
- 12dG. Ung, G. Bertrand, Angew. Chem. Int. Ed. 2013, 52, 11388–11391; Angew. Chem. 2013, 125, 11599–11602.
- 13
- 13aY. Xia, A. S. Dudnik, Y. Li, V. Gevorgyan, Org. Lett. 2010, 12, 5538–5541;
- 13bV. Lavallo, G. D. Frey, S. Kousar, B. Donnadieu, G. Bertrand, Proc. Natl. Acad. Sci. USA 2007, 104, 13569–13573;
- 13cH. Rabaâ, B. Engels, T. Hupp, A. S. K. Hashmi, Int. J. Quantum Chem. 2007, 107, 359–365;
- 13dV. Mamane, P. Hannen, A. Fürstner, Chem. Eur. J. 2004, 10, 4556–4575;
- 13eE. Soriano, J. Marco-Contelles, Organometallics 2006, 25, 4542–4553;
- 13fI. V. Seregin, V. Gevorgya, J. Am. Chem. Soc. 2006, 128, 12050–12051.
- 14
- 14aA. Gómez-Suárez, S. P. Nolan, Angew. Chem. Int. Ed. 2012, 51, 8156–8159; Angew. Chem. 2012, 124, 8278–8281;
- 14bL. Ye, Y. Wang, D. H. Aue, L. Zhang, J. Am. Chem. Soc. 2012, 134, 31–34;
- 14cA. S. K. Hashmi, M. Wieteck, I. Braun, M. Rudolph, F. Rominger, Angew. Chem. Int. Ed. 2012, 51, 10633–10637; Angew. Chem. 2012, 124, 10785–10789;
- 14dA. S. K. Hashmi, M. Wieteck, I. Braun, P. Nösel, L. Jongbloed, M. Rudolph, F. Rominger, Adv. Synth. Catal. 2012, 354, 555–562;
- 14eM. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2013, 52, 2593–2598; Angew. Chem. 2013, 125, 2653–2659;
- 14fI. Braun, A. M. Asiri, A. S. K. Hashmi, ACS Catal. 2013, 3, 1902–1907;
- 14gP. Nösel, T. Lauterbach, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2013, 19, 8634–8641;
- 14hM. M. Hansmann, S. Tsupova, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 2215–2223;
- 14iA. S. K. Hashmi, Acc. Chem. Res. 2014, 47, 864–876;
- 14jJ. Bucher, T. Wurm, K. S. Nalivela, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2014, 53, 3854–3858; Angew. Chem. 2014, 126, 3934–3939;
- 14kH. M. Vilhelmsen, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 1901–1908;
- 14lJ. Xie, C. Pan, A. Abdukadera, C. Zhu, Chem. Soc. Rev. 2014, 43, 5245–5256;
- 14mO. J. S. Pickup, I. Khazal, E. J. Smith, A. C. Whitwood, J. M. Lynam, K. Bolaky, T. C. King, B. W. Rawe, N. Fey, Organometallics 2014, 33, 1751–1761;
- 14nY. Tokimizu, M. Wieteck, M. Rudolph, S. Oishi, N. Fujii, A. S. K. Hashmi, H. Ohno, Org. Lett. 2015, 17, 604–607;
- 14oP. Nösel, V. Müller, S. Mader, S. Moghimi, M. Rudolph, I. Braun, F. Rominger, A. S. K. Hashmi, Adv. Synth. Catal. 2015, 357, 500–506;
- 14pJ. Bucher, T. Stößer, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2015, 54, 1666–1670; Angew. Chem. 2015, 127, 1686–1690;
- 14qP. Morán-Poladura, E. Rubio, J. M. González, Angew. Chem. Int. Ed. 2015, 54, 3052–3055; Angew. Chem. 2015, 127, 3095–3098.
- 15For recent reviews of transition-metal vinylidene complexes, see
- 15aJ. M. Lynam, Chem. Eur. J. 2010, 16, 8238–8247;
- 15bB. M. Trost, A. McClory, Chem. Asian J. 2008, 3, 164–194.
- 16
- 16aM. M. Hansmann, F. Rominger, A. S. K. Hashmi, Chem. Sci. 2013, 4, 1552–1559;
- 16bX.-S. Xiao, W.-L. Kwong, X. Guan, C. Yang, W. Lu, C.-M. Che, Chem. Eur. J. 2013, 19, 9457–9462.
- 17
- 17aJ. B. Lambert, Y. Zhao, H. Wu, J. Org. Chem. 1999, 64, 2729–2736;
- 17bJ. B. Lambert, Y. Zhao, J. Am. Chem. Soc. 1996, 118, 7867–7868.
- 18
- 18aH.-U. Siehl, F.-P. Kaufmann, Y. Apeloig, V. Braude, D. Danovich, A. Berndt, N. Stamatis, Angew. Chem. Int. Ed. Engl. 1991, 30, 1479–1482; Angew. Chem. 1991, 103, 1546–1549;
- 18bH.-U. Siehl, F.-P. Kaufmann, J. Am. Chem. Soc. 1992, 114, 4937–4939;
- 18cH.-U. Siehl, F.-P. Kaufmann, K. Hori, J. Am. Chem. Soc. 1992, 114, 9343–9349;
- 18dH.-U. Siehl, Pure Appl. Chem. 1995, 67, 769–775;
- 18eH.-U. Siehl, S. Brixnera, C. Coletti, N. Reb, B. Chiavarinoc, M. E. Crestonic, A. De Petris, S. Fornarini, Int. J. Mass Spectrom. 2013, 334, 58–66.
- 19T. Müller, R. Meyer, D. Lennartz, H.-U. Siehl, Angew. Chem. Int. Ed. 2000, 39, 3074–3077;
10.1002/1521-3773(20000901)39:17<3074::AID-ANIE3074>3.0.CO;2-T CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 3203–3206.
- 20aH.-U. Steinberger, T. Müller, N. Auner, C. Maerker, P. von R. Schleyer, Angew. Chem. Int. Ed. Engl. 1997, 36, 626–628; Angew. Chem. 1997, 109, 667–669;
- 20bA. Klaer, W. Saak, D. Haase, T. Müller, J. Am. Chem. Soc. 2008, 130, 14956–14957;
- 20cT. Müller, C. Bauch, M. Ostermeier, M. Bolte, N. Auner, J. Am. Chem. Soc. 2003, 125, 2158–2168;
- 20dT. Müller, M. Juhasz, C. A. Reed, Angew. Chem. Int. Ed. 2004, 43, 1543–1546; Angew. Chem. 2004, 116, 1569–1572.
- 21A. Klaer, T. Müller, J. Phys. Org. Chem. 2010, 23, 1043–1048.
- 22T. Müller, D. Margraf, Y. Syha, J. Am. Chem. Soc. 2005, 127, 10852–10860.
- 23A. Klaer, Y. Syha, H. R. Nasiri, T. Müller, Chem. Eur. J. 2009, 15, 8414–8423.
- 24J. B. Lambert, Tetrahedron 1990, 46, 2677–2689.
- 25The length of the alkyl chain connecting the two silicon atoms was critical to successful formation of a stable gold vinylidene complex. For example, hydride abstraction from the (disilylmethyl)acetylide complex [(P)Au{η1-CCSi(Me)2CH2SiMe2H}] formed no detectable vinylidene complex, whereas preliminary investigation of hydride abstraction from the corresponding (disilylpropyl)acetylide complex [(P)Au{η1-C≡CSi(Me)2(CH2)3SiMe2H}] suggested formation of a ground-state π-disilacycloheptyne complex.[28]
- 26
- 26aW. Zhang, J. A. Stone, M. A. Brook, G. A. McGibbon, J. Am. Chem. Soc. 1996, 118, 5764–5771;
- 26bX. Li, J. A. Stone, J. Am. Chem. Soc. 1989, 111, 5586–5592;
- 26cH.-U. Siehl in Dicoordinated Carbocations (Eds.: ), Wiley, New York, 1997, p. 189.
- 27Unfortunately, no correlation between the Δδ and σ+ values has been established for α-aryl-β,β-disilylvinyl cations in which the β-silicon atoms are part of a five-membered ring, such as in 3 and 5. As such, no definitive assignment of the electron-donor ability of the (P)Au fragment of 3 can be determined from these data.
- 28For an example of a neutral gold π-cycloheptyne complex and a cationic π-bis(tert-butyldimethylsilyl)acetylene complex, see
- 28aP. Schulte, U. Behrens, Chem. Commun. 1998, 1633–1634;
- 28bD. Zhu, X. Cao, B. Yu, Org. Chem. Front. 2015, 2, 369–365.