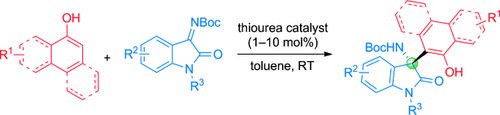
Organocatalytic Asymmetric Addition of Naphthols and Electron-Rich Phenols to Isatin-Derived Ketimines: Highly Enantioselective Construction of Tetrasubstituted Stereocenters†
Marc Montesinos-Magraner
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorDr. Carlos Vila
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorRubén Cantón
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorProf. Dr. Gonzalo Blay
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorDr. Isabel Fernández
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorProf. Dr. M. Carmen Muñoz
Departament de Física Aplicada, Universitat Politècnica de València, Camino de Vera s/n, 46022 València (Spain)
Search for more papers by this authorCorresponding Author
Prof. Dr. José R. Pedro
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)Search for more papers by this authorMarc Montesinos-Magraner
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorDr. Carlos Vila
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorRubén Cantón
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorProf. Dr. Gonzalo Blay
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorDr. Isabel Fernández
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Search for more papers by this authorProf. Dr. M. Carmen Muñoz
Departament de Física Aplicada, Universitat Politècnica de València, Camino de Vera s/n, 46022 València (Spain)
Search for more papers by this authorCorresponding Author
Prof. Dr. José R. Pedro
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)
Departament de Química Orgànica, Facultat de Química, Universitat de València, Dr. Moliner 50, 46100 Burjassot, València (Spain)Search for more papers by this authorFinancial support from the MINECO (Gobierno de España; CTQ2013-47494-P) and from Generalitat Valenciana (ISIC2012/001) is gratefully acknowledged. M.M.-M. thanks the Universitat de València for a predoctoral grant. C.V. thanks MINECO for a JdC contract.
Graphical Abstract
Gentle persuasion: A quinine-derived thiourea organocatalyst was found to promote the asymmetric addition of naphthols and activated phenols to ketimines derived from isatins (see scheme; Boc=tert-butoxycarbonyl). The reaction under mild conditions afforded chiral 3-amino-2-oxindoles containing a quaternary stereocenter in high yields (up to 99 %) with excellent enantioselectivity (up to 99 % ee).
Abstract
A quinine-derived thiourea organocatalyst promoted the highly enantioselective addition of naphthols and activated phenols to ketimines derived from isatins. The reaction afforded chiral 3-amino-2-oxindoles with a quaternary stereocenter in high yields (up to 99 %) with excellent enantioselectivity (up to 99 % ee). To the best of our knowledge, this transformation is the first highly enantioselective addition of naphthols to ketimines.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501273_sm_miscellaneous_information.pdf5.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Kobayashi, Y. Mori, J. S. Fossey, M. M. Salter, Chem. Rev. 2011, 111, 2626–2704;
- 1bG. K. Friestad, A. K. Mathies, Tetrahedron 2007, 63, 2541–2569.
- 2 Chiral Amine Synthesis: Methods, Developments and Applications (Ed.: ), Wiley-VCH, Weinheim, 2010.
- 3
- 3a Friedel–Crafts Chemistry (Ed.: ), Wiley, New York, 1973;
- 3b Catalytic Asymmetric Friedel–Crafts Alkylations (Eds.: ), Wiley-VCH, Weinheim, 2009.
- 4For representative examples, see:
- 4aD. Uraguchi, K. Sorimachi, M. Terada, J. Am. Chem. Soc. 2004, 126, 11804–11805;
- 4bY.-Q. Wang, J. Song, R. Hong, H. Li, L. Deng, J. Am. Chem. Soc. 2006, 128, 8156–8157;
- 4cP. Yu, J. He, C. Guo, Chem. Commun. 2008, 2355–2357;
- 4dQ. Kang, Z.-A. Zhao, S.-L. You, J. Am. Chem. Soc. 2007, 129, 1484–1485;
- 4eM. Terada, S. Yokoyama, K. Sorimachi, D. Uraguchi, Adv. Synth. Catal. 2007, 349, 1863–1867;
- 4fG. B. Rowland, E. B. Rowland, Y. Liang, J. A. Perman, J. C. Antilla, Org. Lett. 2007, 9, 2609–2611;
- 4gM. Terada, K. Sorimachi, J. Am. Chem. Soc. 2007, 129, 292–293;
- 4hG.-W. Zhang, L. Wang, J. Nie, J.-A. Ma, Adv. Synth. Catal. 2008, 350, 1457–1463;
- 4iQ. Kang, X.-J. Zheng, S.-L. You, Chem. Eur. J. 2008, 14, 3539–3542;
- 4jD. Enders, M. Seppelt, T. Beck, Adv. Synth. Catal. 2010, 352, 1413–1418;
- 4kY. Qian, G. Ma, A. Lv, H.-L. Zhu, J. Zhao, V. H. Rawal, Chem. Commun. 2010, 46, 3004–3006;
- 4lM. Johannsen, Chem. Commun. 1999, 2233–2234;
- 4mY. Jia, J. Xie, H. Duan, L. Wang, Q. Zhou, Org. Lett. 2006, 8, 1621–1624;
- 4nL. Liu, Q. Zhao, F. Du, H. Chen, Z. Qin, B. Fu, Tetrahedron: Asymmetry 2011, 22, 1874–1878;
- 4oB. L. Wang, N. K. Li, J. X. Zhang, G. G. Liu, T. Liu, Q. Shen, X. W. Wang, Org. Biomol. Chem. 2011, 9, 2614–2617.
- 5
- 5aY.-X. Jia, J. Zhong, S.-F. Zhu, C.-M. Zhang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2007, 46, 5565–5567; Angew. Chem. 2007, 119, 5661–5663;
- 5bR. Husmann, E. Sugiono, S. Mersmann, G. Raabe, M. Rueping, C. Bolm, Org. Lett. 2011, 13, 1044–1047;
- 5cK.-F. Zhang, J. Nie, R. Guo, Y. Zheng, J.-A. Ma, Adv. Synth. Catal. 2013, 355, 3497–3502;
- 5dM. Rueping, B. J. Nachtsheim, Synlett 2010, 119–122;
- 5eM. Rueping, S. Raja, A. Nuñez, Adv. Synth. Catal. 2011, 353, 563–568;
- 5fJ. Feng, W. Yan, D. Wang, P. Li, Q. Sun, R. Wang, Chem. Commun. 2012, 48, 8003–8005;
- 5gY. Qian, C. Jing, C. Zhai, W.-H. Hu, Adv. Synth. Catal. 2012, 354, 301–307.
- 6
- 6aS. Brandes, M. Bella, A. Kjærsgaard, K. A. Jørgensen, Angew. Chem. Int. Ed. 2006, 45, 1147–1151; Angew. Chem. 2006, 118, 1165–1169;
- 6bS. Brandes, B. Niess, M. Bella, A. Prieto, J. Overgaard, K. A. Jørgensen, Chem. Eur. J. 2006, 12, 6039–6052.
- 7
- 7aD. Wu, X. Zhang, Y. Xu, Y. Xue, J. Li, W. Wang, J. Zhu, Asian J. Org. Chem. 2014, 3, 480–486;
- 7bJ. Kaur, A. Kumar, S. S. Chimni, Tetrahedron Lett. 2014, 55, 2138–2141.
- 8
- 8aT.-Y. Liu, H.-L. Cui, Q. Chai, J. Long, B.-J. Li, Y. Wu, L.-S. Ding, Y.-C. Chen, Chem. Commun. 2007, 2228–2230;
- 8bX.-S. Wang, G.-S. Yang, G. Zhao, Tetrahedron: Asymmetry 2008, 19, 709–714;
- 8cX.-S. Wang, C.-W. Zheng, S.-L. Zhao, Z. Chai, G. Zhao, G.-S. Yang, Tetrahedron: Asymmetry 2008, 19, 2699–2704;
- 8dL. Hong, L. Wang, W. Sun, K. Wong, R. Wang, J. Org. Chem. 2009, 74, 6881–6884;
- 8eY. Sohtome, B. Shin, N. Horitsugi, R. Takagi, K. Noguchi, K. Nagasawa, Angew. Chem. Int. Ed. 2010, 49, 7299–7303; Angew. Chem. 2010, 122, 7457–7461;
- 8fX. Jiang, L. Wu, Y. Xing, L. Wang, S. Wang, Z. Chen, R. Wang, Chem. Commun. 2012, 48, 446–448;
- 8gE. Paradisi, P. Righi, A. Mazzanti, S. Ranieri, G. Bencivenni, Chem. Commun. 2012, 48, 11178–11180;
- 8hL. Yu, X. Xie, S. Wu, R. Wang, W. He, D. Qin, Q. Liu, L. Jing, Tetrahedron Lett. 2013, 54, 3675–3678.
- 9For organocatalytic reactions, see:
- 9aG. Liu, S. Zhang, H. Li, T. Zhang, W. Wang, Org. Lett. 2011, 13, 828–831;
- 9bP. Chauhan, S. S. Chimni, Eur. J. Org. Chem. 2011, 1636–1640;
- 9cS. Takizawa, S. Hirata, K. Murai, H. Fujiokab, H. Sasai, Org. Biomol. Chem. 2014, 12, 5827–5830; for Lewis acid catalyzed reactions, see:
- 9dL.-F. Niu, Y.-C. Xin, R.-L. Wang, F. Jiang, P.-F. Xu, X.-P. Hui, Synlett 2010, 765–768;
- 9eS. Takizawa, F. Arteaga Arteaga, Y. Yoshida, J. Kodera, Y. Nagata, H. Sasai, Dalton Trans. 2013, 42, 11787–11790.
- 10
- 10aI. Szatmári, T. A. Martinek, L. Lázár, F. Fülüp, Eur. J. Org. Chem. 2004, 2231–2238;
- 10bM. Gerlach, C. Maul, PCT Int. Appl. 2001, 94 pp.,WO 2001047866A1 20010705;
- 10cZ. Turgut, E. Pelit, K. Adem, Molecules 2007, 12, 345–352.
- 11
- 11aD.-X. Liu, L.-C. Zhang, Q. Wang, C.-S. Da, Z.-Q. Xin, R. Wang, M. C. K. Choi, A. S. C. Chan, Org. Lett. 2001, 3, 2733–2735;
- 11bC. Cimarelli, G. Palmieri, E. Volpini, Tetrahedron: Asymmetry 2002, 13, 2417–2426;
- 11cJ.-X. Ji, L.-Q. Qiu, C. W. Yip, A. S. C. Chan, J. Org. Chem. 2003, 68, 1589–1590;
- 11dY. Dong, J. Sun, X. Wang, X. Xu, L. Cao, Y. Hu, Tetrahedron: Asymmetry 2004, 15, 1667–1672;
- 11eX. Wang, Y. Dong, J. Sun, X. Xu, R. Li, Y. Hu, J. Org. Chem. 2005, 70, 1897–1900.
- 12
- 12aA. B. Dounay, L. E. Overman, Chem. Rev. 2003, 103, 2945–2964;
- 12bC. Marti, E. M. Carreira, Eur. J. Org. Chem. 2003, 2209–2219;
- 12cC. V. Galliford, K. A. Scheidt, Angew. Chem. Int. Ed. 2007, 46, 8748–8758; Angew. Chem. 2007, 119, 8902–8912;
- 12dF. Zhou, Y. L. Liu, J. Zhou, Adv. Synth. Catal. 2010, 352, 1381–1407;
- 12eJ. E. M. N. Klein, R. J. K. Taylor, Eur. J. Org. Chem. 2011, 6821–6841;
- 12fK. Shen, X. Liu, L. Lin, X. Feng, Chem. Sci. 2012, 3, 327–334.
- 13
- 13aK. Bernard, S. Bogliolo, J. B. Ehrenfeld, Br. J. Pharmacol. 2005, 144, 1037–1050;
- 13bT. Oost, G. Backfisch, S. Bhowmik, M. M. van Gaalen, H. Geneste, W. Hornberger, W. Lubisch, A. Netz, L. Unger, W. Wernet, Bioorg. Med. Chem. Lett. 2011, 21, 3828–3831;
- 13cG. Decaux, A. Soupart, G. Vassart, Lancet 2008, 371, 1624–1632;
- 13dT. Shimazaki, M. Iijima, S. Chaki, Eur. J. Pharmacol. 2006, 543, 63–67.
- 14M. Ochi, K. Kawasaki, H. Kataoka, Y. Uchio, H. Nishi, Biochem. Biophys. Res. Commun. 2001, 283, 1118–1123.
- 15
- 15aM. Rottmann, C. McNamara, B. S. K. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia, J. Tan, S. B. Cohen, K. R. Spencer, G. Gonzalez-Paez, L. Lakshiminarayana, A. Goh, R. Suwanarusk, T. Jegla, E. K. Schmitt, H.-P. Beck, R. Brun, F. Nosten, L. Renia, V. Dartois, T. H. Keller, D. A. Fidock, E. A. Winzeler, T. T. Diagana, Science 2010, 329, 1175–1180;
- 15bB. K. S. Yeung, B. Zou, M. Rottmann, S. B. Lakshminarayana, S. H. Ang, S. Y. Leong, J. Tan, J. Wong, S. Keller-Maerki, C. Fischli, A. Goh, E. K. Schmitt, P. Krastel, E. Francotte, K. Kuhen, D. Plouffe, K. Henson, T. Wagner, E. A. Winzeler, F. Petersen, R. Brun, V. Dartois, T. T. Diagana, T. H. Keller, J. Med. Chem. 2010, 53, 5155–5164.
- 16P. Chauhan, S. S. Chimni, Tetrahedron: Asymmetry 2013, 24, 343–356.
- 17
- 17aL. Cheng, L. Liu, D. Wang, Y.-J. Chen, Org. Lett. 2009, 11, 3874–3877;
- 17bZ.-Q. Qian, F. Zhou, T.-P. Du, B.-L. Wang, M. Ding, X.-L. Zhao, J. Zhou, Chem. Commun. 2009, 6753–6755;
- 17cT. Bui, M. Borregan, C. F. Barbas III, J. Org. Chem. 2009, 74, 8935–8938;
- 17dS. Mouri, Z. Chen, H. Mitsuma, M. Furutachi, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 1255–1257;
- 17eT. Bui, G. Hernandez-Torres, C. Milite, C. F. Barbas III, Org. Lett. 2010, 12, 5696–5699;
- 17fF. Zhou, M. Ding, Y.-L. Liu, C.-H. Wang, C.-B. Ji, Y.-Y. Zhang, J. Zhou, Adv. Synth. Catal. 2011, 353, 2945–2952;
- 17gK. Shen, X. Liu, G. Wang, L. Lin, X. Feng, Angew. Chem. Int. Ed. 2011, 50, 4684–4688; Angew. Chem. 2011, 123, 4780–4784;
- 17hF. Zhou, X.-P. Zeng, C. Wang, X.-L. Zhao, J. Zhou, Chem. Commun. 2013, 49, 2022–2024;
- 17iT. Zhang, L. Cheng, L. Liu, D. Wang, Y.-J. Chen, Tetrahedron: Asymmetry 2010, 21, 2800–2806;
- 17jL.-N. Jia, J. Huang, L. Peng, L.-L. Wang, J.-F. Bai, F. Tian, G.-Y. He, X.-Y. Xu, L.-X. Wang, Org. Biomol. Chem. 2012, 10, 236–239;
- 17kX. Companyó, G. Valero, O. Pineda, T. Calvet, M. Font-Bardía, A. Moyano, R. Rios, Org. Biomol. Chem. 2012, 10, 431–439.
- 18
- 18aN. Hara, S. Nakamura, M. Sano, R. Tamura, Y. Funahashi, N. Shibata, Chem. Eur. J. 2012, 18, 9276–9280;
- 18bW. Yan, D. Wang, J. Feng, P. Li, D. Zhao, R. Wang, Org. Lett. 2012, 14, 2512–2515;
- 18cT.-Z. Li, X.-B. Wang, F. Sha, X.-Y. Wu, J. Org. Chem. 2014, 79, 4332–4339;
- 18dX.-B. Wang, T.-Z. Li, F. Sha, X.-Y. Wu, Eur. J. Org. Chem. 2014, 739–744;
- 18eJ. Zhao, B. Fang, W. Luo, X. Hao, X. Liu, L. Lin, X. Feng, Angew. Chem. Int. Ed. 2015, 54, 241–244; Angew. Chem. 2015, 127, 243–246.
- 19
- 19aY.-L. Lin, F. Zhou, J.-J. Cao, C.-B. Ji, M. Ding, J. Zhou, Org. Biomol. Chem. 2010, 8, 3847–3850;
- 19bD. Wang, J. Liang, J. Feng, K. Wang, Q. Sun, L. Zhao, D. Li, W. Yan, R. Wang, Adv. Synth. Catal. 2013, 355, 548–558;
- 19cY.-L. Liu, J. Zhou, Chem. Commun. 2013, 49, 4421–4423.
- 20
- 20aT. Arai, E. Matsumura, H. Masu, Org. Lett. 2014, 16, 2768–2771;
- 20bM. Holmquist, G. Blay, J. R. Pedro, Chem. Commun. 2014, 50, 9309–9312;
- 20cA. Kumar, J. Kaur, S. S. Chimni, A. K. Jassal, RSC Adv. 2014, 4, 24816–24819;
- 20dY.-H. Wang, Y.-L. Liu, Z.-Y. Cao, J. Zhou, Asian J. Org. Chem. 2014, 3, 429–432.
- 21
- 21aH. Lv, B. Tiwari, J. Mo, C. Xing, Y. R. Chi, Org. Lett. 2012, 14, 5412–5415;
- 21bF.-L. Hu, Y. Wei, M. Shi, S. Pindi, G. Li, Org. Biomol. Chem. 2013, 11, 1921–1924;
- 21cS. Nakamura, K. Hyodo, M. Nakamura, D. Nakane, H. Masuda, Chem. Eur. J. 2013, 19, 7304–7309;
- 21dJ. Xu, C. Mou, T. Zhu, B.-A. Song, Y. R. Chi, Org. Lett. 2014, 16, 3272–3275;
- 21eJ. George, B. Sridhar, V. S. Reddy, Org. Biomol. Chem. 2014, 12, 1595–1602.
- 22
- 22aG. Blay, I. Fernández, A. Monleón, J. R. Pedro, C. Vila, Org. Lett. 2009, 11, 441–444;
- 22bG. Blay, I. Fernández, M. C. Muñoz, A. Recuenco, J. R. Pedro, C. Vila, J. Org. Chem. 2011, 76, 6286–6294.
- 23
- 23a Asymmetric Organocatalysis (Ed.: ), Springer, Berlin, Heidelberg, 2009;
- 23b Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications (Ed.: ), Wiley-VCH, Weinheim, 2013;
- 23c Recent Developments in Asymmetric Organocatalysis (Ed.: ), RSC Publishing, Cambridge, 2010;
- 23d Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2005.
- 24
- 24aS.-K. Tian, Y.-G. Chen, J. F. Hang, L. Tang, P. McDaid, L. Deng, Acc. Chem. Res. 2004, 37, 621–631;
- 24bT. Marcelli, H. Hiemstra, Synthesis 2010, 1229–1279;
- 24cS. Connon, Chem. Eur. J. 2006, 12, 5418–5427;
- 24dJ. Alemán, A. Parra, H. Jiang, K. A. Jørgensen, Chem. Eur. J. 2011, 17, 6890–6899.
- 25For seminal studies on the use of catalyst III for the addition of nucleophiles to imines, see:
- 25aJ. Song, Y. Wang, L. Deng, J. Am. Chem. Soc. 2006, 128, 6048–6049;
- 25bL. Bernardi, F. Fini, R. P. Herrera, A. Ricci, V. Sgarzani, Tetrahedron 2006, 62, 375–380.
- 26The N1-unprotected isatin showed lower reactivity, and the corresponding product was obtained in moderate yield (56 %) with a low ee value (11 %).
- 27See the Supporting Information for further details. CCDC 1048104 (3 p) and 1048103 (5 i) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 28
- 28aP. Chauhan, S. S. Chimni, Tetrahedron Lett. 2013, 54, 4613–4616;
- 28bS. Bai, Y. Liao, L. Lin, W. Luo, X. Liu, X. Feng, J. Org. Chem. 2014, 79, 10662–10668.
- 29One example of the addition of 3-(dimethylamino)phenol to a cyclic ketimine was reported previously;[5c] in that case the product was formed with moderate enantioselectivity (63 % ee).
- 30One example of the addition of 4 a to 2 a is described in Ref. [9 c]; however, the ee value of the product is low (43 %).