Rhodium(III)-Catalyzed [3+2]/[5+2] Annulation of 4-Aryl 1,2,3-Triazoles with Internal Alkynes through Dual C(sp2)H Functionalization†
Yuan Yang
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorMing-Bo Zhou
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorXuan-Hui Ouyang
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorRui Pi
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorDr. Ren-Jie Song
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Heng Li
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000 (China)
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)Search for more papers by this authorYuan Yang
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorMing-Bo Zhou
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorXuan-Hui Ouyang
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorRui Pi
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorDr. Ren-Jie Song
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Heng Li
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000 (China)
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)Search for more papers by this authorWe thank the Natural Science Foundation of China (Nos. 21472039 and 21172060) and Hunan Provincial Natural Science Foundation of China (No. 13JJ2018) for financial support.
Graphical Abstract
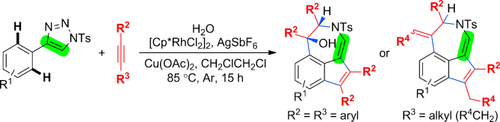
A quantum leap in complexity: A general strategy based on rhodium(III) azavinyl carbene intermediates was established for the oxidative [3+2]/[5+2] annulation of 4-aryl 1-tosyl-1,2,3-triazoles with alkynes. This general method provided densely functionalized indeno[1,7-cd]azepine architectures with excellent selectivity through the functionalization of two C(sp2)H bonds (see scheme; Cp*=pentamethylcyclopentadienyl, Ts= p-toluenesulfonyl).
Abstract
A rhodium(III)-catalyzed [3+2]/[5+2] annulation of 4-aryl 1-tosyl-1,2,3-triazoles with internal alkynes is presented. This transformation provides straightforward access to indeno[1,7-cd]azepine architectures through a sequence involving the formation of a rhodium(III) azavinyl carbene, dual C(sp2)H functionalization, and [3+2]/[5+2] annulation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501260_sm_miscellaneous_information.pdf13.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Kametani, K. Fukumoto, Heterocycles 1975, 3, 931;
- 1bB. Renfroe, C. Harrington, G. R. Proctor, Heterocyclic Compounds: Azepines, Wiley Interscience, New York, 1984;
- 1cC. R. Ganellin, D. J. Triggle, Dictionary of Pharmacological Agents, Chapman & Hall/CRC, London, 1996.
- 2For hainanensine, see:
- 2aN. J. Sun, X. T. Liang, Acta Pharmacol. Sin. 1981, 16, 24;
- 2bD. L. Yin, C. X. Xu, Y. S. Gao, D. K. Liu, S. Y. Wen, J. Y. Guo, Acta Pharmacol. Sin. 1992, 27, 824;
- 2cJ. Y. Guo, D. L. Yin, Y. S. Gao, D. K. Liu, X. T. Liang, Chin. J. Org. Chem. 1993, 13, 195;
- 2dY. M. Ye, C. X. Xu, R. H. Sui, J. Y. Guo, G. J. Cui, Acta Pharmacol. Sin. 1995, 30, 12;
- 2eW. J. Zhang, T. H. Zhou, Acta Pharmacol. Sin. 1998, 33, 212;
- 2fR. D. Clark, K. K. Weinhardt, J. Berger, L. E. Fisher, C. M. Brown, A. C. MacKinnon, A. T. Kilpatrick, M. Spedding, J. Med. Chem. 1990, 33, 633; Cephalotaxine:
- 2gL. Huang, Z. Xue in The Alkaloids, Vol. 23 (Ed.: ), Academic Press, New York, 1984, pp. 157–226;
- 2hT. Hudlicky, L. D. Kwart, J. W. Reed in Alkaloids: Chemical and Biological Perspectives, Vol. 5 (Ed.: ), Wiley, New York, 1987, pp. 639–690;
- 2iM. A. J. Miah, T. Hudlicky, J. W. Reed in The Alkaloids, Vol. 51 (Ed.: ), Academic Press, New York, 1998, pp. 199–269; for zarebradine, see:
- 2jR. E. BoSmith, I. Briggs, N. C. Sturgess, Br. J. Pharmacol. 1993, 110, 343;
- 2kG. Fliss, A. Staab, C. Tillmann, D. Trommeshauser, H. G. Schaefer, C. Kloft, Pharm. Res. 2008, 25, 359;
- 2lS. P. Glasser, D. D. Michie, U. Thadani, W. M. Baiker, Am. J. Cardiol. 1997, 79, 1401; SCH23390:
- 2mL. C. Iorio, A. Barnett, F. H. Leitz, V. P. Houser, C. A. Korduba, Pharmacology 1983, 226, 462; for SKF 38393, see:
- 2nR. G. Pendleton, L. Samler, C. Kaiser, P. T. Ridley, Eur. J. Pharmacol. 1978, 51, 19;
- 2oP. G. Setler, H. M. Sarau, C. L. Zirkle, H. L. Saunders, Eur. J. Pharmacol. 1978, 50, 419; Lorcaserin:
- 2pJ. W. Fleming, K. S. McClendon, D. M. Riche, Ann. Pharmacother. 2013, 47, 1007.
- 3For pioneering studies on the synthesis of azepines by the [5+2] cycloaddition strategy, see:
- 3aE. L. Stogryn, S. J. Brois, J. Am. Chem. Soc. 1967, 89, 605;
- 3bA. Hassner, R. D’Costa, A. T. McPhail, W. Butler, Tetrahedron Lett. 1981, 22, 3691;
- 3cP. A. Wender, T. M. Pedersen, M. J. C. Scanio, J. Am. Chem. Soc. 2002, 124, 15154;
- 3dM.-B. Zhou, R.-J. Song, C.-Y. Wang, J.-H. Li, Angew. Chem. Int. Ed. 2013, 52, 10805; Angew. Chem. 2013, 125, 11005;
- 3eD. J. Lee, H. S. Han, J. Shin, E. J. Yoo, J. Am. Chem. Soc. 2014, 136, 11606.
- 4
- 4aB. Chattopadhyay, V. Gevorgyan, Angew. Chem. Int. Ed. 2012, 51, 862; Angew. Chem. 2012, 124, 886;
- 4bA. V. Gulevich, V. Gevorgyan, Angew. Chem. Int. Ed. 2013, 52, 1371; Angew. Chem. 2013, 125, 1411;
- 4cH. M. L. Davies, J. S. Alford, Chem. Soc. Rev. 2014, 43, 5151.
- 5
- 5aB. T. Parr, S. A. Green, H. M. L. Davies, J. Am. Chem. Soc. 2013, 135, 4716;
- 5bJ. E. Spangler, H. M. L. Davies, J. Am. Chem. Soc. 2013, 135, 6802;
- 5cT. Miura, Y. Funakoshi, M. Murakami, J. Am. Chem. Soc. 2014, 136, 2272;
- 5dJ.-M. Yang, C.-Z. Zhu, X.-Y. Tang, M. Shi, Angew. Chem. Int. Ed. 2014, 53, 5142; Angew. Chem. 2014, 126, 5242;
- 5eX.-Y. Tang, Y.-S. Zhang, L. He, Y. Wei, M. Shi, Chem. Commun. 2015, 51, 133;
- 5fS. Park, W.-S. Yong, S. Kim, P. H. Lee, Org. Lett. 2014, 16, 4468;
- 5gB. Rajagopal, C.-H. Chou, C.-C. Chung, P.-C. Lin, Org. Lett. 2014, 16, 3752.
- 6For other selected examples of the use of 1-sulfonyl-1,2,3-triazoles as precursors of RhII azavinyl carbenes in synthesis, see:
- 6aS. Chuprakov, F. W. Hwang, V. Gevorgyan, Angew. Chem. Int. Ed. 2007, 46, 4757; Angew. Chem. 2007, 119, 4841;
- 6bT. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan, V. V. Fokin, J. Am. Chem. Soc. 2008, 130, 14972;
- 6cT. Miura, M. Yamauchi, M. Murakami, Chem. Commun. 2009, 1470;
- 6dM. Zibinsky, V. V. Fokin, Angew. Chem. Int. Ed. 2013, 52, 1507; Angew. Chem. 2013, 125, 1547;
- 6eS. Chuprakov, S. W. Kwok, V. V. Fokin, J. Am. Chem. Soc. 2013, 135, 4652;
- 6fB. T. Parr, H. M. L. Davies, Angew. Chem. Int. Ed. 2013, 52, 10044; Angew. Chem. 2013, 125, 10228;
- 6gJ. S. Alford, J. E. Spangler, H. M. L. Davies, J. Am. Chem. Soc. 2013, 135, 11712;
- 6hT. Miura, K. Hiraga, T. Biyajima, T. Nakamuro, M. Murakami, Org. Lett. 2013, 15, 3298;
- 6iY. Shi, V. Gevorgyan, Org. Lett. 2013, 15, 5394;
- 6jS. Chuprakov, B. T. Worrell, N. Selander, R. K. Sit, V. V. Fokin, J. Am. Chem. Soc. 2014, 136, 195;
- 6kS. W. Kwok, L. Zhang, N. P. Grimster, V. V. Fokin, Angew. Chem. Int. Ed. 2014, 53, 3452; Angew. Chem. 2014, 126, 3520;
- 6lC. Kim, S. Park, D. Eom, B. Seo, P. H. Lee, Org. Lett. 2014, 16, 1900;
- 6mD. J. Jung, H. J. Jeon, J. H. Kim, Y. Kim, S. Lee, Org. Lett. 2014, 16, 2208;
- 6nH. Shang, Y. Wang, Y. Tian, J. Feng, Y. Tang, Angew. Chem. Int. Ed. 2014, 53, 5662; Angew. Chem. 2014, 126, 5768.
- 7For selected reviews, see:
- 7aT. Satoh, M. Miura, Synthesis 2010, 3395;
- 7bT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212;
- 7cF. W. Patureau, J. Wencel-Delord, F. Glorius, Aldrichimica Acta 2012, 45, 31;
- 7dG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651; for representative examples of rhodium(III)-catalyzed aryl C(sp2)H annulation with alkynes in the presence of oxidants, see:
- 7eD. R. Stuart, M. Bertrand-Laperle, K. M. N. Burgess, K. Fagnou, J. Am. Chem. Soc. 2008, 130, 16474;
- 7fN. Guimond, K. Fagnou, J. Am. Chem. Soc. 2009, 131, 12050;
- 7gT. Fukutani, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Commun. 2009, 5141;
- 7hK. Morimoto, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2010, 12, 2068;
- 7iF. W. Patureau, T. Besset, N. Kuhl, F. Glorius, J. Am. Chem. Soc. 2011, 133, 2154;
- 7jK. Muralirajan, K. Parthasarathy, C.-H. Cheng, Angew. Chem. Int. Ed. 2011, 50, 4169; Angew. Chem. 2011, 123, 4255;
- 7kJ. Jayakumar, K. Parthasarathy, C.-H. Cheng, Angew. Chem. Int. Ed. 2012, 51, 197; Angew. Chem. 2012, 124, 201;
- 7lB.-J. Li, H.-Y. Wang, Q.-L. Zhu, Z.-J. Shi, Angew. Chem. Int. Ed. 2012, 51, 3948; Angew. Chem. 2012, 124, 4014;
- 7mX. Tan, B. Liu, X. Li, B. Li, S. Xu, H. Song, B. Wang, J. Am. Chem. Soc. 2012, 134, 16163;
- 7nJ. Jayakumar, K. Parthasarathy, Y.-H. Chen, T.-H. Lee, S.-C. Chuang, C.-H. Cheng, Angew. Chem. Int. Ed. 2014, 53, 9889; Angew. Chem. 2014, 126, 10047;
- 7oM.-B. Zhou, R. Pi, M. Hu, Y. Yang, R.-J. Song, Y. Xia, J.-H. Li, Angew. Chem. Int. Ed. 2014, 53, 11338; Angew. Chem. 2014, 126, 11520;
- 7pS. Yu, X. Li, Org. Lett. 2014, 16, 1200;
- 7qK. Morimoto, M. Itoh, K. Hirano, T. Satoh, Y. Shibata, K. Tanaka, M. Miura, Angew. Chem. Int. Ed. 2012, 51, 5359; Angew. Chem. 2012, 124, 5455;
- 7rY. Chen, F. Wang, W. Zhen, X. Li, Adv. Synth. Catal. 2013, 355, 353.
- 8CCDC 1048349 (4 gf) and CCDC 1048350 (8 aa) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The structure of diastereoisomers 3 was determined by 2D NMR spectroscopic analysis of 3 aa and from the single-crystal structure of 8 aa (see Supporting Information).
- 9V. Narasimhan, R. Rathore, S. Chandrasekaran, Synth. Commun. 1985, 15, 769.
- 10
- 10aA. J. C. Walters, E. Jellema, M. Finger, P. Aarnoutse, J. M. M. Smits, J. N. H. Reek, B. de Bruin, ACS Catal. 2012, 2, 246;
- 10bS. Yu, S. Liu, Y. Lan, B. Wan, X. Li, J. Am. Chem. Soc. 2015, 137, 1623.