Carboxylic-Acid-Passivated Metal Oxide Nanocrystals: Ligand Exchange Characteristics of a New Binding Motif†
This work was financially supported by the Research Foundation Flanders (FWO), the Strategic Initiative Materials (SIM-Flanders), Ghent University, and the Hercules Foundation.
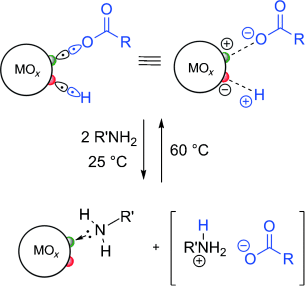
Graphical Abstract
Abstract
Ligand exchange is central in the processing of inorganic nanocrystals (NCs) and requires understanding of surface chemistry. Studying sterically stabilized HfO2 and ZrO2 NCs using 1H solution NMR and IR spectroscopy as well as elemental analysis, this paper demonstrates the reversible exchange of initial oleic acid ligands for octylamine and self-adsorption of oleic acid at NC surfaces. Both processes are incompatible with an X-type binding motif of carboxylic acids as reported for sulfide and selenide NCs. We argue that this behavior stems from the dissociative adsorption of carboxylic acids at the oxide surface. Both proton and carboxylate moieties must be regarded as X-type ligands yielding a combined X2 binding motif that allows for self-adsorption and exchange for L-type ligands.