Benzannulation of Triynes to Generate Functionalized Arenes by Spontaneous Incorporation of Nucleophiles†
Rajdip Karmakar
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Search for more papers by this authorDr. Sang Young Yun
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Search for more papers by this authorJiajia Chen
College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang Province 325035 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Yuanzhi Xia
College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang Province 325035 (P.R. China)
Yuanzhi Xia, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang Province 325035 (P.R. China)
Daesung Lee, Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Search for more papers by this authorCorresponding Author
Prof. Daesung Lee
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Yuanzhi Xia, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang Province 325035 (P.R. China)
Daesung Lee, Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Search for more papers by this authorRajdip Karmakar
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Search for more papers by this authorDr. Sang Young Yun
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Search for more papers by this authorJiajia Chen
College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang Province 325035 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Yuanzhi Xia
College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang Province 325035 (P.R. China)
Yuanzhi Xia, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang Province 325035 (P.R. China)
Daesung Lee, Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Search for more papers by this authorCorresponding Author
Prof. Daesung Lee
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Yuanzhi Xia, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang Province 325035 (P.R. China)
Daesung Lee, Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607 (USA)
Search for more papers by this authorWe thank UIC (LAS-AFS, DL), NSF (CHE-1361620, DL), TACOMA Technology (DL), the Zhejiang Provincial NSF (LY13B020007, YX), and the NNSFC (21372178, YX) for financial support. We thank Prof. Neal Mankad for his help in obtaining X-ray structures. All computations were carried out on the High Performance Computation Platform of Wenzhou University. The mass spectrometry facility at UIUC is greatly acknowledged.
Graphical Abstract
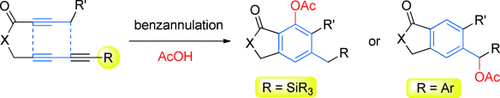
Small but profound: In the benzannulation reaction of 1,3,8-triynes, a small structural difference has a profound impact on the structure of the products. When R is a silyl group, a nucleophile is incorporated into the newly formed benzene core, whereas when R is an aryl group, nucleophile trapping occurs at the benzylic carbon atom connected to the aryl group.
Abstract
The thermal reaction of ester-tethered 1,3,8-triynes provides novel benzannulation products with concomitant incorporation of a nucleophile. Evidence suggests that this reaction proceeds via an allene-enyne intermediate generated by an Alder-ene reaction in the first step. Depending on the substituent of the alkyne moiety on the allene-enyne intermediate, the subsequent transformation can take one of two different paths, each leading to discrete aromatization products. The benzannulation of a silane-substituted 1,3,8-triynes provides arene products with a nucleophile incorporated onto the newly formed benzene core, whereas an aryl substituent leads to nucleophile trapping at the benzylic carbon atom connected to the aryl substituent. The formation of these two different products results from the involvement of two regioisomeric allene-enyne intermediates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201412468_sm_miscellaneous_information.pdf7.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. R. Jones, R. G. Bergman, J. Am. Chem. Soc. 1972, 94, 660–661;
- 1bK. H. Dötz, Angew. Chem. Int. Ed. Engl. 1975, 14, 644–645; Angew. Chem. 1975, 87, 672–673;
- 1cR. G. Bergman, Acc. Chem. Res. 1973, 6, 25–31;
- 1dR. L. Danheiser, S. K. Gee, J. Org. Chem. 1984, 49, 1672–1674;
- 1eA. G. Myers, E. Y. Kuo, N. S. Finney, J. Am. Chem. Soc. 1989, 111, 8057–8059;
- 1fR. L. Danheiser, R. G. Brisbois, J. J. Kowalczyk, R. F. Miller, J. Am. Chem. Soc. 1990, 112, 3093–3100;
- 1gA. G. Myers, P. S. Dragovich, E. Y. Kuo, J. Am. Chem. Soc. 1992, 114, 9369–9386;
- 1hR. Nagata, H. Yamanaka, E. Okazaki, I. Saito, Tetrahedron Lett. 1989, 30, 4995–4998;
- 1iY. Wang, M. G. Finn, J. Am. Chem. Soc. 1995, 117, 8045–8046;
- 1jK. Yoshida, T. Imamoto, J. Am. Chem. Soc. 2005, 127, 10470–10471;
- 1kF. Pünner, G. Hilt, Chem. Commun. 2012, 48, 3617–3619;
- 1lK. Yamada, M. J. Lear, T. Yamaguchi, S. Yamashita, I. D. Gridnev, Y. Hayashi, M. Hirama, Angew. Chem. Int. Ed. 2014, 53, 13902–13906; Angew. Chem. 2014, 126, 14122–14126;
- 1mP. García-García, M. A. Fernández-Rodríguez, E. Aguilar, Angew. Chem. Int. Ed. 2009, 48, 5534–5537; Angew. Chem. 2009, 121, 5642–5645;
- 1nW. T. Teo, W. Rao, C. J. H. Ng, S. W. Y. Koh, P. W. H. Chan, Org. Lett. 2014, 16, 1248–1251; For reviews, see:
- 1oS. Saito, Y. Yamamoto, Chem. Rev. 2000, 100, 2901–2915;
- 1pA. Basak, S. Mandal, S. S. Bag, Chem. Rev. 2003, 103, 4077–4094;
- 1qS. Kotha, S. Misra, S. Halder, Tetrahedron 2008, 64, 10775–10790;
- 1rR. K. Mohamed, P. W. Peterson, I. V. Alabugin, Chem. Rev. 2013, 113, 7089–7129.
- 2
- 2aA. Z. Bradley, R. P. Johnson, J. Am. Chem. Soc. 1997, 119, 9917–9918;
- 2bK. Miyawaki, R. Suzuki, T. Kawano, I. Ueda, Tetrahedron Lett. 1997, 38, 3943–3946;
- 2cT. R. Hoye, B. Baire, D. Niu, P. H. Willoughby, B. P. Woods, Nature 2012, 490, 208–212;
- 2dS. Y. Yun, K.-P. Wang, N.-K. Lee, P. Mamidipalli, D. Lee, J. Am. Chem. Soc. 2013, 135, 4668–4671; For a review see
- 2eC. Holden, M. F. Greaney, Angew. Chem. Int. Ed. 2014, 53, 5746–5749; Angew. Chem. 2014, 126, 5854–5857.
- 3R. Karmakar, S. Y. Yun, K.-P. Wang, D. Lee, Org. Lett. 2014, 16, 6–9.
- 4The Grubbs second-generation complex is used as a catalyst when AcOH is the trapping agent, wherein we believe the ruthenium complexes catalyzes the isomerization of B to B′ in Scheme 2. Although the role of these complexes is yet to be further elucidated, it generally reduces the formation of unknown by-products. See the Supporting Information for details.
- 5The structures of 3 d (CCDC 1040777), 3 l (CCDC 1040778), and 4 h′′ (CCDC 1040779) were confirmed by X-ray diffraction analysis. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 6A methanol adduct similar to those described in Scheme 3 was isolated in 39 % yield. See the Supporting Information for details.
- 7All reported energy values are relative free energies including solvation corrections. See the Supporting Information for details.
- 8Y. Zhao, D. G. Truhlar, Acc. Chem. Res. 2008, 41, 157–167.
- 9Intramolecular propargylic ene reaction, see:
- 9aD. Peña, D. Pérez, E. Guitián, L. Castedo, Eur. J. Org. Chem. 2003, 2003, 1238–1243;
- 9bM. Altable, S. Filippone, A. Martín-Domenech, M. Güell, M. Solà, N. Martín, Org. Lett. 2006, 8, 5959–5962;
- 9cI. González, A. Pla-Quintana, A. Roglans, A. Dachs, M. Solaà‘, T. Parella, J. Farjas, P. Roura, V. Lloveras, J. Vidal-Gancedo, Chem. Commun. 2010, 46, 2944–2946;
- 9dJ. M. Robinson, T. Sakai, K. Okano, T. Kitawaki, R. L. Danheiser, J. Am. Chem. Soc. 2010, 132, 11039–11041;
- 9eT. Sakai, R. L. Danheiser, J. Am. Chem. Soc. 2010, 132, 13203–13205;
- 9fY. Lan, R. L. Danheiser, K. N. Houk, J. Org. Chem. 2012, 77, 1533–1538.
- 10A higher barrier (TSc=15.7 kcal mol−1) is calculated for more stable triplet biradical (−33.4 kcal mol−1).
- 11For a radical-based justification for the reaction of allene and alkyne moieties, see: M. R. Siebert, J. M. Osbourn, M. K. Brummond, D. J. Tantillo, J. Am. Chem. Soc. 2010, 132, 11952–11966.
- 12For heteroatom nucleophile attack on allenic carbon atom, see:
- 12aT. Sugita, M. Eida, H. Ito, N. Komatsu, K. Abe, M. Suama, J. Org. Chem. 1987, 52, 3789–3793;
- 12bH. Liu, W. Feng, C. W. Kee, D. Leow, W.-T. Loh, C.-H. Tan, Adv. Synth. Catal. 2010, 352, 3373–3379;
- 12cG. Kumaraswamy, N. Jayaprakash, G. Balakishan, Org. Biomol. Chem. 2011, 9, 7913–7920;
- 12dG. Chen, C. Fu, S. Ma, Org. Lett. 2009, 11, 2900–2903;
- 12eW. H. Pecak, J. Son, A. J. Burnstine, L. L. Anderson, Org. Lett. 2014, 16, 3440–3443; For a review, see:
- 12fS. Kitagaki, T. Kawamura, D. Shibata, C. Mukai, Tetrahedron 2008, 64, 11086–11095.
- 13The Michael addition of HOAc to B′ is 1.4 kcal mol−1 higher in energy than TS1.
- 14
- 14aW. Reischl, W. H. Okamura, J. Am. Chem. Soc. 1982, 104, 6115–6117;
- 14bY. W. Andemichael, K. K. Wang, J. Org. Chem. 1992, 57, 796–798;
- 14cT. Choshi, T. Sada, H. Fujimoto, C. Nagayama, E. Sugino, S. Hibino, Tetrahedron Lett. 1996, 37, 2593–2596;
- 14dD. Ghosh, S. Jana, A. Panja, A. Anoop, A. Basak, Tetrahedron 2013, 69, 8724–8730;
- 14eH. Zhou, Y. Xing, J. Yao, Y. Lu, J. Org. Chem. 2011, 76, 4582–4590;
- 14fG. Zhao, Q. Zhang, H. Zhou, Adv. Synth. Catal. 2013, 355, 3492–3496;
- 14gC. Zhu, S. Ma, Org. Lett. 2014, 16, 1542–1545. For a review on electrocyclization, see:
- 14hC. M. Beaudry, J. P. Malerich, D. Trauner, Chem. Rev. 2005, 105, 4757–4778.
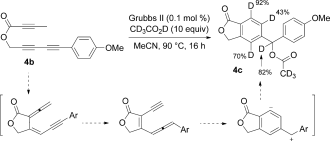
- 15The mechanism for the formation these products is yet to be elucidated but at least it is not consistent with the pathway involving B→B′→D, based on the unusually high calculated activation barrier (72.6 kcal mol−1) and the deuterium incorporation pattern in 4 c. Relying on several lines of evidence, we tentatively propose that with an aryl substituent, B′ favorably rearranges to a regioisomeric allene-enyne (a consequence of a formal [1,7]-H shift), which then undergoes an ionic Saito–Myers cyclization to generate a zwitterionic intermediate, the trapping of which provides the observed product.
- 16The compound 6 a′ does not have a long enough lifetime for 13C NMR spectroscopy, and it readily converts into 6 a.
- 17Similarly, the methanol adduct mentioned in Ref. [6] was converted into the corresponding benzannulated product under three different conditions. See the Supporting Information for details.
- 18
- 18aA. D. Ketley, J. L. McClanah, J. Org. Chem. 1965, 30, 942–943;
- 18bH. K. Sonawane, B. S. Nanjundiah, G. M. Nazeruddin, Tetrahedron Lett. 1992, 33, 1645–1646;
- 18cP. Binger, P. Wedemann, S. I. Kozhushkov, A. De Meijere, Eur. J. Org. Chem. 1998, 1998, 113–120. For reviews, see:
- 18dJ. E. Baldwin, Chem. Rev. 2003, 103, 1197–1212;
- 18eT. Hudlicky, J. W. Reed, Angew. Chem. Int. Ed. 2010, 49, 4864–4876; Angew. Chem. 2010, 122, 4982–4994.