Highlight
Building Up Quarternary Stereocenters of Chromans by Asymmetric Redox Organocatalysis: A New Entry to Vitamin E
Dr. Thomas Netscher,
Corresponding Author
Dr. Thomas Netscher
Research and Development, DSM Nutritional Products, P.O. Box 2676, 4002 Basel (Switzerland)
Research and Development, DSM Nutritional Products, P.O. Box 2676, 4002 Basel (Switzerland)Search for more papers by this authorDr. Thomas Netscher,
Corresponding Author
Dr. Thomas Netscher
Research and Development, DSM Nutritional Products, P.O. Box 2676, 4002 Basel (Switzerland)
Research and Development, DSM Nutritional Products, P.O. Box 2676, 4002 Basel (Switzerland)Search for more papers by this authorGraphical Abstract
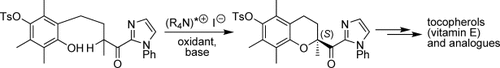
High-turnover catalysis offers a novel concept for the efficient chemo- and enantioselective preparation of chroman intermediates, which are useful for the synthesis of tocopherols (vitamin E components) and other biologically active compounds. A chiral ammonium iodide catalyst mediates the cycloetherification in combination with a cooxidant and an inorganic base in excellent yield and up to 93 % ee. OTs=para-toluenesulfonyl.
References
- 1
- 1aH. C. Shen, Tetrahedron 2009, 65, 3931–3952;
- 1bM. G. Núñez, P. García, R. F. Moro, D. Díez, Tetrahedron 2010, 66, 2089–2109;
- 1cM. L. Colombo, Molecules 2010, 15, 2103–2113;
- 1dT. Netscher, Vitam. Horm. 2007, 76, 155–202 and references therein.
- 2aG. W. Burton, K. U. Ingold, Acc. Chem. Res. 1986, 19, 194–201;
- 2bJ. Weimann, H. Weiser, Am. J. Clin. Nutr. 1991, 53, 1056S–1060S;
- 2cH. Weiser, M. Vecchi, Int. J. Vit. Nutr. Res. 1982, 52, 351–370.
- 3
- 3aR. Noyori, Angew. Chem. Int. Ed. 2002, 41, 2008–2022;
10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2108–2123;
- 3bR. Noyori, H. Takaya, Acc. Chem. Res. 1990, 23, 345–350;
- 3cT. Netscher, M. Scalone, R. Schmid in Asymmetric Catalysis on Industrial Scale (Eds.: ), Wiley-VCH, Weinheim, 2004, pp. 71–89;
- 3dD. H. Woodmansee, A. Pfaltz, Chem. Commun. 2011, 47, 7912–7916;
- 3eD. H. Woodmansee, A. Pfaltz, Top. Organomet. Chem. 2011, 34, 31–76.
- 4
- 4aB. M. Trost, J. Org. Chem. 2004, 69, 5813–5837;
- 4bB. M. Trost, H. C. Shen, L. Dong, J.-P. Surivet, C. Sylvain, J. Am. Chem. Soc. 2004, 126, 11966–11983;
- 4cB. M. Trost, F. D. Toste, J. Am. Chem. Soc. 1998, 120, 9074–9075.
- 5
- 5aL. F. Tietze, F. Strecker, J. Zinngrebe, K. M. Sommer, Chem. Eur. J. 2006, 12, 8770–8776;
- 5bL. F. Tietze, K. M. Sommer, J. Zinngrebe, F. Strecker, Angew. Chem. Int. Ed. 2005, 44, 257–259; Angew. Chem. 2005, 117, 262–264;
- 5cfor the catalytic asymmetric Wacker-type cyclization to chromans see: Y. Uozumi, K. Kato, T. Hayashi, J. Am. Chem. Soc. 1997, 119, 5063–5064.
- 6
- 6a Science of Synthesis: Asymmetric Organocatalysis (Eds.: ), Georg Thieme, Stuttgart, 2012;
- 6b Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2007;
- 6cA. Dondoni, A. Massi, Angew. Chem. Int. Ed. 2008, 47, 4638–4660; Angew. Chem. 2008, 120, 4716–4739;
- 6dA. Moyano, R. Rios, Chem. Rev. 2011, 111, 4703–4832.
- 7
- 7aA. Chougnet, K. Liu, W.-D. Woggon, Chimia 2010, 64, 303–308;
- 7bK. Liu, A. Chougnet, W.-D. Woggon, Angew. Chem. Int. Ed. 2008, 47, 5827–5829; Angew. Chem. 2008, 120, 5911–5913.
- 8
- 8aW.-D. Woggon, Angew. Chem. 1999, 111, 2881–2883;
10.1002/(SICI)1521-3757(19990917)111:18<2881::AID-ANGE2881>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2715–2716;10.1002/(SICI)1521-3773(19990917)38:18<2715::AID-ANIE2715>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 8bD. Shintani, D. DellaPenna, Science 1998, 282, 2098–2100.
- 9
- 9aM. Uyanik, H. Hayashi, K. Ishihara, Science 2014, 345, 291–294;
- 9bB. J. Nachtsheim, Science 2014, 345, 270–271;
- 9cS. Borman, Chem. Eng. News 2014, 92( 29), 8; for earlier work on this topic see:
- 9dM. Uyanik, H. Okamoto, T. Yasui, K. Ishihara, Science 2010, 328, 1376–1379;
- 9eA. N. French, Science 2010, 328, 1365–1366;
- 9fS. Everts, Chem. Eng. News 2010, 88( 24), 11.
- 10T. Ooi, K. Maruoka, Angew. Chem. Int. Ed. 2007, 46, 4222–4266; Angew. Chem. 2007, 119, 4300–4345.