Influence of the β-Sheet Content on the Mechanical Properties of Aggregates during Amyloid Fibrillization†
Francesco Simone Ruggeri
Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
These authors contributed equally.
Search for more papers by this authorDr. Jozef Adamcik
Food and Soft Materials Science, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, LFO E23, 8092, Zürich (Switzerland)
These authors contributed equally.
Search for more papers by this authorJae Sun Jeong
Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
Search for more papers by this authorProf. Hilal A. Lashuel
Laboratory of Molecular and Chemical Biology of Neurodegeneration, Brain Mind Institute, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Raffaele Mezzenga
Food and Soft Materials Science, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, LFO E23, 8092, Zürich (Switzerland)
Raffaele Mezzenga, Food and Soft Materials Science, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, LFO E23, 8092, Zürich (Switzerland)
Giovanni Dietler, Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Giovanni Dietler
Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
Raffaele Mezzenga, Food and Soft Materials Science, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, LFO E23, 8092, Zürich (Switzerland)
Giovanni Dietler, Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
Search for more papers by this authorFrancesco Simone Ruggeri
Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
These authors contributed equally.
Search for more papers by this authorDr. Jozef Adamcik
Food and Soft Materials Science, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, LFO E23, 8092, Zürich (Switzerland)
These authors contributed equally.
Search for more papers by this authorJae Sun Jeong
Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
Search for more papers by this authorProf. Hilal A. Lashuel
Laboratory of Molecular and Chemical Biology of Neurodegeneration, Brain Mind Institute, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Raffaele Mezzenga
Food and Soft Materials Science, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, LFO E23, 8092, Zürich (Switzerland)
Raffaele Mezzenga, Food and Soft Materials Science, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, LFO E23, 8092, Zürich (Switzerland)
Giovanni Dietler, Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Giovanni Dietler
Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
Raffaele Mezzenga, Food and Soft Materials Science, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, LFO E23, 8092, Zürich (Switzerland)
Giovanni Dietler, Laboratory of Physics of Living Matter, Ecole Polytechnique Fédérale de Lausanne (EPFL), Route de la Sorge, 1015 Lausanne (Switzerland)
Search for more papers by this authorWe thank the Swiss National Foundation for Science for financial support (Project Number 152958).
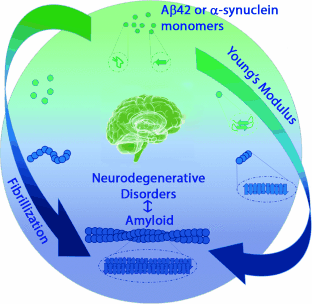
Graphical Abstract
Inflexibility that comes with age: During amyloid fibrillization, which is associated with neurodegenerative disorders, initially formed oligomeric and protofibrillar species aggregate to form fibrils with a core cross-β-sheet structure (see picture). AFM peak force quantitative nanomechanical measurements revealed an increase in the Young's modulus during the fibrillization process in conjunction with an increase in the amyloid β-sheet content.
Abstract
Amyloid fibrils associated with neurodegenerative diseases, such as Parkinson’s and Alzheimer’s, consist of insoluble aggregates of α-synuclein and Aβ-42 proteins with a high β-sheet content. The aggregation of both proteins occurs by misfolding of the monomers and proceeds through the formation of intermediate oligomeric and protofibrillar species to give the final fibrillar cross-β-sheet structure. The morphological and mechanical properties of oligomers, protofibrils, and fibrils formed during the fibrillization process were investigated by thioflavin T fluorescence and circular dichroism in combination with AFM peak force quantitative nanomechanical technique. The results reveal an increase in the Young’s modulus during the transformation from oligomers to mature fibrils, thus inferring that the difference in their mechanical properties is due to an internal structural change from a random coil to a structure with increased β-sheet content.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201409050_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. Caughey, P. T. Lansbury, Annu. Rev. Neurosci. 2003, 26, 267–298.
- 2C. M. Dobson, Nature 2003, 426, 884–890.
- 3F. Chiti, C. M. Dobson, Annu. Rev. Biochem. 2006, 75, 333–366.
- 4M. R. Chapman, L. S. Robinson, J. S. Pinkner, R. Roth, J. Heuser, M. Hammar, S. Normark, S. J. Hultgren, Science 2002, 295, 851–855.
- 5D. M. Fowler, A. V. Koulov, W. E. Balch, J. W. Kelly, Trends Biochem. Sci. 2007, 32, 217–224.
- 6F. Shewmaker, R. P. McGlinchey, R. B. Wickner, J. Biol. Chem. 2011, 286, 16533–16540.
- 7M. Sunde, L. C. Serpell, M. Bartlam, P. E. Fraser, M. B. Pepys, C. C. Blake, J. Mol. Biol. 1997, 273, 729–739.
- 8R. Nelson, M. R. Sawaya, M. Balbirnie, A. Ø. Madsen, C. Riekel, R. Grothe, D. Eisenberg, Nature 2005, 435, 773–778.
- 9A. W. P. Fitzpatrick, G. T. Debelouchina, M. J. Bayro, D. K. Clare, M. A. Caporini, V. S. Bajaj, C. P. Jaroniec, L. Wang, V. Ladizhansky, S. A. Müller, C. E. MacPhee, C. A. Waudby, H. R. Mott, A. De Simone, T. P. J. Knowles, H. R. Saibil, M. Vendruscolo, E. V. Orlova, R. G. Griffin, C. M. Dobson, Proc. Natl. Acad. Sci. USA 2013, 110, 5468–5473.
- 10J. Adamcik, R. Mezzenga, Macromolecules 2012, 45, 1137–1150.
- 11J. F. Smith, T. P. Knowles, C. M. Dobson, C. E. Macphee, M. E. Welland, Proc. Natl. Acad. Sci. USA 2006, 103, 15806–15811.
- 12T. P. Knowles, A. W. Fitzpatrick, S. Meehan, H. R. Mott, M. Vendruscolo, C. M. Dobson, M. E. Welland, Science 2007, 318, 1900–1903.
- 13T. P. Knowles, M. J. Buehler, Nat. Nanotechnol. 2011, 6, 469–479.
- 14A. W. Fitzpatrick, S. T. Park, A. H. Zewail, Proc. Natl. Acad. Sci. USA 2013, 110, 10976–10981.
- 15C. Li, J. Adamcik, R. Mezzenga, Nat. Nanotechnol. 2012, 7, 421–427.
- 16T. P. Knowles, T. W. Oppenheim, A. K. Buell, D. Y. Chirgadze, M. E. Welland, Nat. Nanotechnol. 2010, 5, 204–207.
- 17S. Ling, C. Li, J. Adamcik, Z. Shao, X. Chen, R. Mezzenga, Adv. Mater. 2014, 26, 4569–4574.
- 18I. Cherny, E. Gazit, Angew. Chem. Int. Ed. 2008, 47, 4062–4069; Angew. Chem. 2008, 120, 4128–4136.
- 19L. Adler-Abramovich, N. Kol, I. Yanai, D. Barlam, R. Z. Shneck, E. Gazit, I. Rousso, Angew. Chem. Int. Ed. 2010, 49, 9939–9942; Angew. Chem. 2010, 122, 10135–10138.
- 20J. Adamcik, J. M. Jung, J. Flakowski, P. De Los Rios, G. Dietler, R. Mezzenga, Nat. Nanotechnol. 2010, 5, 423–428.
- 21A. Relini, S. Torrassa, R. Ferrando, R. Rolandi, S. Campioni, F. Chiti, A. Gliozzi, Biophys. J. 2010, 98, 1277–1284.
- 22J. Adamcik, R. Mezzenga, Curr. Opin. Colloid Interface Sci. 2012, 17, 369–376.
- 23Z. Xu, R. Paparcone, M. J. Buehler, Biophys. J. 2010, 98, 2053–2062.
- 24R. Paparcone, M. J. Buehler, Biomaterials 2011, 32, 3367–3374.
- 25R. Paparcone, S. Keten, M. J. Buehler, J. Biomech. 2010, 43, 1196–1201.
- 26R. Paparcone, S. W. Cranford, M. J. Buehler, Nanoscale 2011, 3, 1748–1755.
- 27M. Solar, M. J. Buehler, J. Mech. Behav. Biomed. Mater. 2013, 19, 43–49.
- 28M. Solar, M. J. Buehler, Nanotechnology 2014, 25, 105703.
- 29J. Adamcik, A. Berquand, R. Mezzenga, Appl. Phys. Lett. 2011, 98, 193701.
- 30J. Adamcik, C. Lara, I. Usov, J. S. Jeong, F. S. Ruggeri, G. Dietler, H. A. Lashuel, I. W. Hamley, R. Mezzenga, Nanoscale 2012, 4, 4426–4429.
- 31C. Lara, S. Gourdin-Bertin, J. Adamcik, S. Bolisetty, R. Mezzenga, Biomacromolecules 2012, 13, 4213–4221.
- 32K. Sweers, K. van der Werf, M. Bennink, V. Subramaniam, Nanoscale Res. Lett. 2011, 6, 270.
- 33L. Giehm, N. Lorenzen, D. E. Otzen, Methods 2011, 53, 295–305.
- 34M. Calero, M. Gasset, Methods Mol. Biol. 2012, 849, 53–68.
- 35A. L. Fink, Acc. Chem. Res. 2006, 39, 628–634.
- 36J. S. Jeong, A. Ansaloni, R. Mezzenga, H. A. Lashuel, G. Dietler, J. Mol. Biol. 2013, 425, 1765–1781.
- 37J. S. Pedersen, C. B. Andersen, D. E. Otzen, FEBS J. 2010, 277, 4591–4601.
- 38C. Haass, D. J. Selkoe, Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112.
- 39D. Hong, S. Han, A. L. Fink, V. N. Uversky, Protein Pept. Lett. 2011, 18, 230–240.