Construction of Axial Chirality by Rhodium-Catalyzed Asymmetric Dehydrogenative Heck Coupling of Biaryl Compounds with Alkenes†
Jun Zheng
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Collaborative Innovation Center of Chemical Science and Engineering (Tianjin)
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/Search for more papers by this authorJun Zheng
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Collaborative Innovation Center of Chemical Science and Engineering (Tianjin)
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/Search for more papers by this authorWe thank the National Basic Research Program of China (973 Program 2015CB856600) and the National Natural Science Foundation of China (21025209, 21121062, and 21332009) for generous financial support.
Graphical Abstract
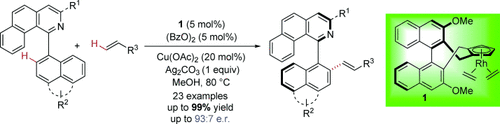
An axe to grind? Novel axially chiral biaryls were synthesized by the direct CH bond olefination of biaryl compounds, using a chiral [Cp*RhIII] catalyst (1), in good to excellent yields and enantioselectivities. The biaryls were found as suitable ligands for rhodium-catalyzed asymmetric conjugate addition reactions.
Abstract
Enantioselective construction of axially chiral biaryls by direct CH bond functionalization reactions has been realized. Novel axially chiral biaryls were synthesized by the direct CH bond olefination of biaryl compounds, using a chiral [Cp*RhIII] catalyst, in good to excellent yields and enantioselectivities. The obtained axially chiral biaryls were found as suitable ligands for rhodium-catalyzed asymmetric conjugate additions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201408805_sm_miscellaneous_information.pdf5.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a book, see: G. Bringmann, C. Günther, M. Ochse, O. Schupp, S. Tasler in Progress in the Chemistry of Organic Natural Products, Vol. 82 (Eds.: ), Springer, Vienna, 2001, pp. 1–249.
- 2For reviews, see:
- 2aC. Rosini, L. Franzini, A. Raffaelli, P. Salvadori, Synthesis 1992, 503–517;
- 2bL. Pu, Chem. Rev. 1998, 98, 2405–2494;
- 2cM. McCarthy, P. J. Guiry, Tetrahedron 2001, 57, 3809–3844;
- 2dH. Shimizu, I. Nagasaki, T. Saito, Tetrahedron 2005, 61, 5405–5432;
- 2eT. Akiyama, J. Itoh, K. Fuchibe, Adv. Synth. Catal. 2006, 348, 999–1010;
- 2fY.-M. Li, F.-Y. Kwong, W.-Y. Yu, A. S. C. Chan, Coord. Chem. Rev. 2007, 251, 2119–2144;
- 2gY. Canac, R. Chauvin, Eur. J. Inorg. Chem. 2010, 2325–2335.
- 3For selected reviews, see:
- 3aN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457–2483;
- 3bO. Baudoin, Eur. J. Org. Chem. 2005, 4223–4229;
- 3cK. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. Int. Ed. 2005, 44, 4442–4489; Angew. Chem. 2005, 117, 4516–4563;
- 3dF. Alonso, I. P. Beletskaya, M. Yus, Tetrahedron 2008, 64, 3047–3101.
- 4For selected asymmetric desymmetrization of prochiral biaryl compounds, see:
- 4aT. Harada, T. M. T. Tuyet, A. Oku, Org. Lett. 2000, 2, 1319–1322;
- 4bT. M. T. Tuyet, T. Harada, K. Hashimoto, M. Hatsuda, A. Oku, J. Org. Chem. 2000, 65, 1335–1343;
- 4cY.-Y. Ku, T. Grieme, P. Raje, P. Sharma, S. A. King, H. E. Morton, J. Am. Chem. Soc. 2002, 124, 4282–4286;
- 4dY.-H. Cho, A. Kina, T. Shimada, T. Hayashi, J. Org. Chem. 2004, 69, 3811–3823.
- 5For reviews, see:
- 5aG. Bringmann, M. Breuning, S. Tasler, Synthesis 1999, 525–558;
- 5bG. Bringmann, D. Menche, Acc. Chem. Res. 2001, 34, 615–624.
- 6For a review, see:
- 6aH. Wang, Chirality 2010, 22, 827–837. For selected examples of asymmetric oxidative coupling of 2-naphthol, see:
- 6bZ. Luo, Q. Liu, L. Gong, X. Cui, A. Mi, Y. Jiang, Angew. Chem. Int. Ed. 2002, 41, 4532–4535;
10.1002/1521-3773(20021202)41:23<4532::AID-ANIE4532>3.0.CO;2-5 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 4714–4717;
- 6cZ. Luo, Q. Liu, L. Gong, X. Cui, A. Mi, Y. Jiang, Chem. Commun. 2002, 914–915;
- 6dC. A. Mulrooney, X. Li, E. S. DiVirgilio, M. C. Kozlowski, J. Am. Chem. Soc. 2003, 125, 6856–6857;
- 6eQ.-X. Guo, Z.-J. Wu, Z.-B. Luo, Q.-Z. Liu, J.-L. Ye, S.-W. Luo, L.-F. Cun, L.-Z. Gong, J. Am. Chem. Soc. 2007, 129, 13927–13938.
- 7For a review on atroposelective [2+2+2] cycloaddition, see:
- 7aK. Tanaka, Chem. Asian J. 2009, 4, 508–518; For selected examples, see:
- 7bT. Shibata, T. Fujimoto, K. Yokota, K. Takagi, J. Am. Chem. Soc. 2004, 126, 8382–8383;
- 7cT. Suda, K. Noguchi, M. Hirano, K. Tanaka, Chem. Eur. J. 2008, 14, 6593–6596;
- 7dJ. Oppenheimer, W. L. Johnson, R. Figueroa, R. Hayashi, R. P. Hsung, Tetrahedron 2009, 65, 5001–5012;
- 7eA. Mori, T. Araki, Y. Miyauchi, K. Noguchi, K. Tanaka, Eur. J. Org. Chem. 2013, 6774–6778.
- 8For reviews on asymmetric CH functionalization, see:
- 8aR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242–3272;
- 8bL. Yang, H. Huang, Catal. Sci. Technol. 2012, 2, 1099–1112;
- 8cJ. Wencel-Delord, F. Colobert, Chem. Eur. J. 2013, 19, 14010–14017;
- 8dC. Zheng, S.-L. You, RSC Adv. 2014, 4, 6173–6214; For selected reviews on rhodium/(III)-catalyzed CH functionalization, see:
- 8eT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212–11222;
- 8fJ. Wencel-Delord, T. Dröge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740–4761;
- 8gC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215–1292;
- 8hD. A. Colby, A. S. Tsai, R. G. Bergman, J. A. Ellman, Acc. Chem. Res. 2012, 45, 814–825;
- 8iG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651–3678;
- 8jN. Kuhl, N. Schröder, F. Glorius, Adv. Synth. Catal. 2014, 356, 1443–1460;
- 8kX.-S. Zhang, K. Chen, Z.-J. Shi, Chem. Sci. 2014, 5, 2146–2159.
- 9F. Kakiuchi, P. Le Gendre, A. Yamada, H. Ohtaki, S. Murai, Tetrahedron: Asymmetry 2000, 11, 2647–2651.
- 10K. Yamaguchi, J. Yamaguchi, A. Studer, K. Itami, Chem. Sci. 2012, 3, 2165–2169.
- 11K. Yamaguchi, H. Kondo, J. Yamaguchi, K. Itami, Chem. Sci. 2013, 4, 3753–3757.
- 12T. Wesch, F. R. Leroux, F. Colobert, Adv. Synth. Catal. 2013, 355, 2139–2144.
- 13For a recent report on palladium-catalyzed intermolecular direct CH bond iodination of biaryl compounds by kinetic resolution from our group, see: D.-W. Gao, Q. Gu, S.-L. You, ACS Catal. 2014, 4, 2741–2745.
- 14For selected examples on steric hindered CH bond functionalization reaction of biaryl compounds, see:
- 14aN. Chatani, T. Uemura, T. Asaumi, Y. Ie, F. Kakiuchi, S. Murai, Can. J. Chem. 2005, 83, 755–763;
- 14bC.-G. Feng, M. Ye, K.-J. Xiao, S. Li, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 9322–9325.
- 15T. K. Hyster, L. Knörr, T. R. Ward, T. Rovis, Science 2012, 338, 500–503.
- 16
- 16aB. Ye, N. Cramer, Science 2012, 338, 504–506;
- 16bB. Ye, N. Cramer, J. Am. Chem. Soc. 2013, 135, 636–639;
- 16cB. Ye, P. A. Donets, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 507–511; Angew. Chem. 2014, 126, 517–521;
- 16dB. Ye, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 7896–7899; Angew. Chem. 2014, 126, 8030–8033.
- 17G. Song, W. W. N. O, Z. Hou, J. Am. Chem. Soc. 2014, 136, 12209–12212.
- 18CCDC 1022013 (rac-1 be) and 1022014 1 be (enantiopure) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19For representative examples on self-disproportionation of enantiomers, see:
- 19aV. A. Soloshonok, Angew. Chem. Int. Ed. 2006, 45, 766–769; Angew. Chem. 2006, 118, 780–783;
- 19bV. A. Soloshonok, H. Ueki, M. Yasumoto, S. Mekala, J. S. Hirschi, D. A. Singleton, J. Am. Chem. Soc. 2007, 129, 12112–12113.
- 20For leading reviews, see:
- 20aF. Glorius, Angew. Chem. Int. Ed. 2004, 43, 3364–3366; Angew. Chem. 2004, 116, 3444–3446;
- 20bJ. B. Johnson, T. Rovis, Angew. Chem. Int. Ed. 2008, 47, 840–871; Angew. Chem. 2008, 120, 852–884;
- 20cC. Defieber, H. Grützmacher, E. M. Carreira, Angew. Chem. Int. Ed. 2008, 47, 4482–4502; Angew. Chem. 2008, 120, 4558–4579;
- 20dR. Shintani, T. Hayashi, Aldrichimica Acta 2009, 42, 31–38;
- 20eC.-G. Feng, M.-H. Xu, G.-Q. Lin, Synlett 2011, 1345–1356;
- 20fP. Tian, H.-Q. Dong, G.-Q. Lin, ACS Catal. 2012, 2, 95–119;
- 20gX. Feng, H. Du, Asian J. Org. Chem. 2012, 1, 204–213.