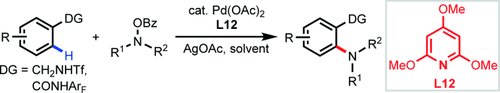
Ligand-Promoted ortho-CH Amination with Pd Catalysts†
Dr. Dajian Zhu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Guoqiang Yang
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorJian He
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorLing Chu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Gang Chen
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Wei Gong
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Ke Chen
Chemical Development, 1 Squibb Drive, New Brunswick, NJ 08903 (USA)
Search for more papers by this authorDr. Martin D. Eastgate
Chemical Development, 1 Squibb Drive, New Brunswick, NJ 08903 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)Search for more papers by this authorDr. Dajian Zhu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Guoqiang Yang
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorJian He
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorLing Chu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Gang Chen
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Wei Gong
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Ke Chen
Chemical Development, 1 Squibb Drive, New Brunswick, NJ 08903 (USA)
Search for more papers by this authorDr. Martin D. Eastgate
Chemical Development, 1 Squibb Drive, New Brunswick, NJ 08903 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)Search for more papers by this authorWe gratefully acknowledge The Scripps Research Institute, and the U.S. NSF (CHE-1011898) for financial support. D.Z. is a visiting scholar from Huazhong University of Science and Technology, sponsored by the China Scholarship Council (201206165031). G.Y. thanks the Shanghai Jiao Tong University for HaiWai ShiZi ChuBei postdoctoral fellowship support.
Graphical Abstract
Trimethoxylpyridine is an efficient ligand for promoting Pd-catalyzed ortho-CH amination of both benzamides and triflyl-protected benzylamines. This finding provides guidance for the development of ligands that can improve or enable PdII-catalyzed C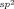 H activation reactions directed by weakly coordinating functional groups.
H activation reactions directed by weakly coordinating functional groups.
Abstract
2,4,6-Trimethoxypyridine is identified as an efficient ligand for promoting a Pd-catalyzed ortho-CH amination of both benzamides and triflyl-protected benzylamines. This finding provides guidance for the development of ligands that can improve or enable PdII-catalyzed C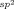 H activation reactions directed by weakly coordinating functional groups.
H activation reactions directed by weakly coordinating functional groups.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201408651_sm_miscellaneous_information.pdf2.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews of CH amination, see:
- 1aH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417;
- 1bK. M. Engle, T.-S. Mei, X. Wang, J.-Q. Yu, Angew. Chem. Int. Ed. 2011, 50, 1478; Angew. Chem. 2011, 123, 1514;
- 1cV. S. Thirunavukkarasu, S. I. Kozhushkov, L. Ackermann, Chem. Commun. 2014, 50, 29.
- 2For selected examples of Pd-catalyzed intramolecular CH amidation, see:
- 2aW. C. P. Tsang, N. Zheng, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 14560;
- 2bM. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2008, 130, 14058;
- 2cT.-S. Mei, X. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 10806;
- 2dG. T. Rice, M. C. White, J. Am. Chem. Soc. 2009, 131, 11707;
- 2eJ. J. Neumann, S. Rakshit, T. Dröge, F. Glorius, Angew. Chem. Int. Ed. 2009, 48, 6892; Angew. Chem. 2009, 121, 7024;
- 2fG. He, Y. Zhao, S. Zhang, C. Lu, G. Chen, J. Am. Chem. Soc. 2012, 134, 3;
- 2gE. T. Nadres, O. Daugulis, J. Am. Chem. Soc. 2012, 134, 7;
- 2hT.-S. Mei, D. Leow, H. Xiao, B. N. Laforteza, J.-Q. Yu, Org. Lett. 2013, 15, 3058;
- 2iG. He, S.-Y. Zhang, W. A. Nack, Q. Li, G. Chen, Angew. Chem. Int. Ed. 2013, 52, 11124; Angew. Chem. 2013, 125, 11330;
- 2jQ. Zhang, K. Chen, W. Rao, Y. Zhang, F.-J. Chen, B.-F. Shi, Angew. Chem. Int. Ed. 2013, 52, 13588; Angew. Chem. 2013, 125, 13833.
- 3For a selected example of Pd-catalyzed intramolecular CH amination, see: J. A. Jordan-Hore, C. C. C. Johansson, M. Gulias, E. M. Beck, M. J. Gaunt, J. Am. Chem. Soc. 2008, 130, 16184.
- 4For a rare example of Pd-catalyzed intramolecular CH activation/CN bond forming reactions with oxime esters, see: Y. Tan, J. F. Hartwig, J. Am. Chem. Soc. 2010, 132, 3676.
- 5For selected examples of Pd-catalyzed intermolecular CH amidation, see:
- 5aH.-Y. Thu, W.-Y. Yu, C.-M. Che, J. Am. Chem. Soc. 2006, 128, 9048;
- 5bK.-H. Ng, A. S. C. Chan, W.-Y. Yu, J. Am. Chem. Soc. 2010, 132, 12862;
- 5cG. Yin, Y. Wu, G. Liu, J. Am. Chem. Soc. 2010, 132, 11978;
- 5dB. Xiao, T.-J. Gong, J. Xu, Z.-J. Liu, L. Liu, J. Am. Chem. Soc. 2011, 133, 1466;
- 5eÁ. Iglesias, R. Álvarez, Á. R. de Lera, K. Muñiz, Angew. Chem. Int. Ed. 2012, 51, 2225; Angew. Chem. 2012, 124, 2268;
- 5fR. Shrestha, P. Mukherjee, Y. Tan, Z. C. Litman, J. F. Hartwig, J. Am. Chem. Soc. 2013, 135, 8480.
- 6For selected examples of Pd-catalyzed intermolecular CH amination, see:
- 6aE. J. Yoo, S. Ma, T.-S. Mei, K. S. L. Chan, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 7652;
- 6bJ. Pan, M. Su, S. L. Buchwald, Angew. Chem. Int. Ed. 2011, 50, 8647; Angew. Chem. 2011, 123, 8806;
- 6cZ. Dong, G. Dong, J. Am. Chem. Soc. 2013, 135, 18350.
- 7For selected examples of Rh-catalyzed CH amidation, see:
- 7aD. E. Olson, J. Du Bois, J. Am. Chem. Soc. 2008, 130, 11248;
- 7bF.-N. Ng, Z. Zhou, W.-Y. Yu, Chem. Eur. J. 2014, 20, 4474.
- 8For selected examples of Rh-catalyzed CH amination, see:
- 8aC. Grohmann, H. Wang, F. Glorius, Org. Lett. 2012, 14, 656;
- 8bK. Wu, Z. Fan, Y. Xue, Q. Yao, A. Zhang, Org. Lett. 2014, 16, 42.
- 9For selected examples of Rh-catalyzed CH activation/CN bond forming reactions using aryl azides as the nitrogen sources, see:
- 9aQ. Nguyen, K. Sun, T. G. Driver, J. Am. Chem. Soc. 2012, 134, 7262;
- 9bJ. Ryu, K. Shin, S. H. Park, J. Y. Kim, S. Chang, Angew. Chem. Int. Ed. 2012, 51, 9904; Angew. Chem. 2012, 124, 10042.
- 10For selected examples of Cu-catalyzed CH activation/CN bond forming reactions, see:
- 10aX. Chen, X.-S. Hao, C. E. Goodhue, J.-Q. Yu, J. Am. Chem. Soc. 2006, 128, 6790;
- 10bH. Wang, Y. Wang, C. Peng, J. Zhang, Q. Zhu, J. Am. Chem. Soc. 2010, 132, 13217;
- 10cS. H. Cho, J. Yoon, S. Chang, J. Am. Chem. Soc. 2011, 133, 5996;
- 10dL. D. Tran, J. Roane, O. Daugulis, Angew. Chem. Int. Ed. 2013, 52, 6043; Angew. Chem. 2013, 125, 6159;
- 10eZ. Wang, J. Ni, Y. Kuninobu, M. Kanai, Angew. Chem. Int. Ed. 2014, 53, 3496; Angew. Chem. 2014, 126, 3564;
- 10fX. Wu, Y. Zhao, G. Zhang, H. Ge, Angew. Chem. Int. Ed. 2014, 53, 3706; Angew. Chem. 2014, 126, 3780;
- 10gM. Shang, S.-Z. Sun, H.-X. Dai, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 3354.
- 11For selected examples of Ru-catalyzed CH activation/CN bond forming reactions, see:
- 11aJ. Kim, J. Kim, S. Chang, Chem. Eur. J. 2013, 19, 7328;
- 11bM. R. Yadav, R. K. Rit, A. K. Sahoo, Org. Lett. 2013, 15, 1638;
- 11cM. Shang, S.-H. Zeng, S.-Z. Sun, H.-X. Dai, J.-Q. Yu, Org. Lett. 2013, 15, 5286.
- 12For selected examples of Ir-catalyzed CH activation/CN bond forming reactions, see:
- 12aJ. Kim, S. Chang, Angew. Chem. Int. Ed. 2014, 53, 2203; Angew. Chem. 2014, 126, 2235;
- 12bL. J. Allen, P. J. Cabrera, M. Lee, M. S. Sanford, J. Am. Chem. Soc. 2014, 136, 5607;
- 12cH. Kim, K. Shin, S. Chang, J. Am. Chem. Soc. 2014, 136, 5904.
- 13For selected examples of Co-catalyzed CH amidation, see:
- 13aH. Lu, H. Jiang, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2010, 49, 10192; Angew. Chem. 2010, 122, 10390;
- 13bH. Lu, C. Li, H. Jiang, C. L. Lizardi, X. P. Zhang, Angew. Chem. Int. Ed. 2014, 53, 7028; Angew. Chem. 2014, 126, 7148.
- 14For selected examples of Fe-catalyzed CH activation/CN bond forming reactions, see:
- 14aS. M. Paradine, M. C. White, J. Am. Chem. Soc. 2012, 134, 2036;
- 14bQ. Nguyen, T. Nguyen, T. G. Driver, J. Am. Chem. Soc. 2013, 135, 620;
- 14cT. Matsubara, S. Asako, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2014, 136, 646.
- 15For selected examples of Rh-catalyzed CH activation and annulation for CN bond forming reactions, see:
- 15aH. Wang, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 7318; Angew. Chem. 2012, 124, 7430;
- 15bG. Zhang, L. Yang, Y. Wang, Y. Xie, H. Huang, J. Am. Chem. Soc. 2013, 135, 8850;
- 15cC. Wang, H. Sun, Y. Fang, Y. Huang, Angew. Chem. Int. Ed. 2013, 52, 5795; Angew. Chem. 2013, 125, 5907;
- 15dJ. Jayakumar, K. Parthasarathy, Y.-H. Chen, T.-H. Lee, S.-C. Chuang, C.-H. Cheng, Angew. Chem. Int. Ed. 2014, 53, 9889; Angew. Chem. 2014, 126, 10047.
- 16For selected examples of other metal-catalyzed CH activation and annulation for CN bond forming reactions, see:
- 16aY.-F. Wang, X. Zhu, S. Chiba, J. Am. Chem. Soc. 2012, 134, 3679;
- 16bU. Sharma, R. Kancherla, T. Naveen, S. Agasti, D. Maiti, Angew. Chem. Int. Ed. 2014, 53, 11895; Angew. Chem. 2014, 126, 12089;
- 16cR. He, Z.-T. Huang, Q.-Y. Zheng, C. Wang, Angew. Chem. Int. Ed. 2014, 53, 4950; Angew. Chem. 2014, 126, 5050.
- 17
- 17aJ. P. Wolfe, S. Wagaw, S. L. Buchwald, J. Am. Chem. Soc. 1996, 118, 7215;
- 17bM. S. Driver, J. F. Hartwig, J. Am. Chem. Soc. 1996, 118, 7217;
- 17cD. W. Old, J. P. Wolfe, S. L. Buchwald, J. Am. Chem. Soc. 1998, 120, 9722.
- 18
- 18aB.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. Int. Ed. 2008, 47, 4882; Angew. Chem. 2008, 120, 4960;
- 18bD.-H. Wang, K. M. Engle, B.-F. Shi, J.-Q. Yu, Science 2010, 327, 315;
- 18cK. M. Engle, D.-H. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 14137.
- 19
- 19aM. Wasa, K. S. L. Chan, X.-G. Zhang, J. He, M. Miura, J.-Q. Yu, J. Am. Chem. Soc. 2012, 134, 18570;
- 19bJ. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homs, J.-Q. Yu, Science 2014, 343, 1216;
- 19cS. Li, G. Chen, C.-G. Feng, W. Gong, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 5267;
- 19dY. Deng, W. Gong, J. He, J.-Q. Yu, Angew. Chem. Int. Ed. 2014, 53, 6692; Angew. Chem. 2014, 126, 6810.
- 20L. Chu, X.-C. Wang, C. E. Moore, A. L. Rheingold, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 16344.