A Natural-Product Switch for a Dynamic Protein Interface†
Marcel Scheepstra
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorDr. Lidia Nieto
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorDr. Anna K. H. Hirsch
Stratingh Institue for Chemistry, University of Groningen, Nijenborgh 7, 9747AG Groningen (The Netherlands)
Search for more papers by this authorDr. Sascha Fuchs
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorDr. Seppe Leysen
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorChan Vinh Lam
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorLeslie in het Panhuis
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorProf. Dr. Constant A. A. van Boeckel
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorDr. Hans Wienk
Bijvoet Center for Biomolecular Research, NMR Spectroscopy, Utrecht University, Padualaan 8, 3584CH Utrecht (The Netherlands)
Search for more papers by this authorProf. Dr. Rolf Boelens
Bijvoet Center for Biomolecular Research, NMR Spectroscopy, Utrecht University, Padualaan 8, 3584CH Utrecht (The Netherlands)
Search for more papers by this authorDr. Christian Ottmann
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorCorresponding Author
Dr. Lech-Gustav Milroy
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cbSearch for more papers by this authorCorresponding Author
Prof. Dr. Luc Brunsveld
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cbSearch for more papers by this authorMarcel Scheepstra
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorDr. Lidia Nieto
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorDr. Anna K. H. Hirsch
Stratingh Institue for Chemistry, University of Groningen, Nijenborgh 7, 9747AG Groningen (The Netherlands)
Search for more papers by this authorDr. Sascha Fuchs
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorDr. Seppe Leysen
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorChan Vinh Lam
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorLeslie in het Panhuis
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorProf. Dr. Constant A. A. van Boeckel
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorDr. Hans Wienk
Bijvoet Center for Biomolecular Research, NMR Spectroscopy, Utrecht University, Padualaan 8, 3584CH Utrecht (The Netherlands)
Search for more papers by this authorProf. Dr. Rolf Boelens
Bijvoet Center for Biomolecular Research, NMR Spectroscopy, Utrecht University, Padualaan 8, 3584CH Utrecht (The Netherlands)
Search for more papers by this authorDr. Christian Ottmann
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Search for more papers by this authorCorresponding Author
Dr. Lech-Gustav Milroy
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cbSearch for more papers by this authorCorresponding Author
Prof. Dr. Luc Brunsveld
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cb
Laboratory of Chemical Biology and Institute of Complex Molecular Systems (ICMS), Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612 AZ Eindhoven (The Netherlands) http://www.tue.nl/cbSearch for more papers by this authorWe thank Nicky Hoek, Dr. Eric Kalkhoven and Dr. Arjen Koppen (UMC, Utrecht, The Netherlands) for their scientific support. Funding was granted by the Netherlands Organisation for Scientific Research via Gravity program 024.001.035, ECHO grant 711011017, VENI grant to A.K.H.H., and a Marie Curie Action (PIEF-GA-2011-298489 to LN).
Graphical Abstract
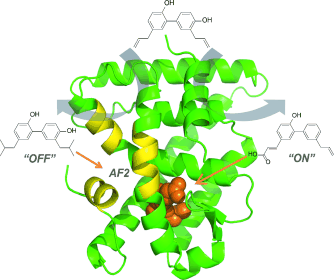
Rational splitting of the dual-binding properties of a natural product delivers two mechanistically different ligands, which selectively target opposite sides of a dynamic protein interface (see picture; AF2=activation function 2). This work highlights the value of screening natural products against transient protein complexes.
Abstract
Small ligands are a powerful way to control the function of protein complexes via dynamic binding interfaces. The classic example is found in gene transcription where small ligands regulate nuclear receptor binding to coactivator proteins via the dynamic activation function 2 (AF2) interface. Current ligands target the ligand-binding pocket side of the AF2. Few ligands are known, which selectively target the coactivator side of the AF2, or which can be selectively switched from one side of the interface to the other. We use NMR spectroscopy and modeling to identify a natural product, which targets the retinoid X receptor (RXR) at both sides of the AF2. We then use chemical synthesis, cellular screening and X-ray co-crystallography to split this dual activity, leading to a potent and molecularly efficient RXR agonist, and a first-of-kind inhibitor selective for the RXR/coactivator interaction. Our findings justify future exploration of natural products at dynamic protein interfaces.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403773_sm_miscellaneous_information.pdf7.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Y. Majmudar, J. W. Højfeldt, C. J. Arevang, W. C. Pomerantz, J. K. Gagnon, P. J. Schultz, L. C. Cesa, C. H. Doss, S. P. Rowe, V. Vásquez, et al., Angew. Chem. 2012, 124, 11420–11424;
10.1002/ange.201206815 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11258–11262;
- 1bN. Wang, C. Y. Majmudar, W. C. Pomerantz, J. K. Gagnon, J. D. Sadowsky, J. L. Meagher, T. K. Johnson, J. A. Stuckey, C. L. Brooks, J. A. Wells, A. K. Mapp, J. Am. Chem. Soc. 2013, 135, 3363–3366.
- 2P. Huang, V. Chandra, F. Rastinejad, Annu. Rev. Physiol. 2010, 72, 247–272.
- 3J.-M. Wurtz, W. Bourguet, J.-P. Renaud, V. Vivat, P. Chambon, D. Moras, H. Gronemeyer, Nat. Struct. Mol. Biol. 1996, 3, 87–94.
- 4L. Nagy, J. W. R. Schwabe, Trends Biochem. Sci. 2004, 29, 317–324.
- 5
- 5aJ. T. Moore, J. L. Collins, K. H. Pearce, ChemMedChem 2006, 1, 504–523.
- 6
- 6aT. W. Moore, C. G. Mayne, J. A. Katzenellenbogen, Mol. Endocrinol. 2010, 24, 683–695;
- 6bJ. H. Choi, A. S. Banks, T. M. Kamenecka, S. A. Busby, M. J. Chalmers, N. Kumar, D. S. Kuruvilla, Y. Shin, Y. He, J. B. Bruning, et al., Nature 2011, 477, 477–481;
- 6cP. Sadana, Future Med. Chem. 2012, 4, 1307–1333.
- 7C. L. Smith, B. W. O’Malley, Endocr. Rev. 2004, 25, 45–71.
- 8
- 8aT. R. Geistlinger, R. K. Guy, J. Am. Chem. Soc. 2003, 125, 6852–6853;
- 8bC. Phillips, L. R. Roberts, M. Schade, R. Bazin, A. Bent, N. L. Davies, R. Moore, A. D. Pannifer, A. R. Pickford, S. H. Prior, et al., J. Am. Chem. Soc. 2011, 133, 9696–9699.
- 9
- 9aV. Azzarito, K. Long, N. S. Murphy, A. J. Wilson, Nat. Chem. 2013, 5, 161–173;
- 9bL. A. Arnold, E. Estébanez-Perpiñá, M. Togashi, N. Jouravel, A. Shelat, A. C. McReynolds, E. Mar, P. Nguyen, J. D. Baxter, R. J. Fletterick, et al., J. Biol. Chem. 2005, 280, 43048–43055;
- 9cL. A. Arnold, A. Kosinski, E. Estébanez-Perpiñá, R. J. Fletterick, R. K. Guy, J. Med. Chem. 2007, 50, 5269–5280;
- 9dJ. Becerril, A. D. Hamilton, Angew. Chem. 2007, 119, 4555–4557;
10.1002/ange.200700657 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4471–4473;
- 9eJ. R. Gunther, A. A. Parent, J. A. Katzenellenbogen, ACS Chem. Biol. 2009, 4, 435–440;
- 9fP. Nandhikonda, W. Z. Lynt, M. M. McCallum, T. Ara, A. M. Baranowski, N. Y. Yuan, D. Pearson, D. D. Bikle, R. K. Guy, L. A. Arnold, J. Med. Chem. 2012, 55, 4640–4651;
- 9gA. L. Rodriguez, A. Tamrazi, M. L. Collins, J. A. Katzenellenbogen, J. Med. Chem. 2004, 47, 600–611;
- 9hE. Estébanez-Perpiñá, L. A. Arnold, A. A. Arnold, P. Nguyen, E. D. Rodrigues, E. Mar, R. Bateman, P. Pallai, K. M. Shokat, J. D. Baxter, et al., Proc. Natl. Acad. Sci. USA 2007, 104, 16074–16079;
- 9iA. Sun, T. W. Moore, J. R. Gunther, M.-S. Kim, E. Rhoden, Y. Du, H. Fu, J. P. Snyder, J. A. Katzenellenbogen, ChemMedChem 2011, 6, 654–666;
- 9jL. Caboni, G. K. Kinsella, F. Blanco, D. Fayne, W. N. Jagoe, M. Carr, D. C. Williams, M. J. Meegan, D. G. Lloyd, J. Med. Chem. 2012, 55, 1635–1644;
- 9kA. L. LaFrate, J. R. Gunther, K. E. Carlson, J. A. Katzenellenbogen, Bioorg. Med. Chem. 2008, 16, 10075–10084.
- 10B. Over, S. Wetzel, C. Grütter, Y. Nakai, S. Renner, D. Rauh, H. Waldmann, Nat. Chem. 2013, 5, 21–28.
- 11The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC.
- 12A. R. de Lera, W. Bourguet, L. Altucci, H. Gronemeyer, Nat. Rev. Drug Discovery 2007, 6, 811–820.
- 13
- 13aV. Jayalakshmi, N. R. Krishna, J. Magn. Reson. 2002, 155, 106–118;
- 13bN. R. Krishna, V. Jayalakshmi, Top. Curr. Chem. 2008, 273, 15–54.
- 14K. T. Liby, M. M. Yore, M. B. Sporn, Nat. Rev. Cancer 2007, 7, 357–369.
- 15L. Altucci, M. D. Leibowitz, K. M. Ogilvie, A. R. de Lera, H. Gronemeyer, Nat. Rev. Drug Discovery 2007, 6, 793–810.
- 16P. E. Cramer, J. R. Cirrito, D. W. Wesson, C. Y. D. Lee, J. C. Karlo, A. E. Zinn, B. T. Casali, J. L. Restivo, W. D. Goebel, M. J. James, et al., Science 2012, 335, 1503–1506.
- 17J. H. Barnard, J. C. Collings, A. Whiting, S. A. Przyborski, T. B. Marder, Chem. Eur. J. 2009, 15, 11430–11442.
- 18
- 18aH. Kotani, H. Tanabe, H. Mizukami, M. Makishima, M. Inoue, J. Nat. Prod. 2010, 73, 1332–1336;
- 18bC.-G. Jung, H. Horike, B.-Y. Cha, K.-O. Uhm, R. Yamauchi, T. Yamaguchi, T. Hosono, K. Iida, J.-T. Woo, M. Michikawa, Biol. Pharm. Bull. 2010, 33, 1105–1111.
- 19Y.-J. Lee, Y. M. Lee, C.-K. Lee, J. K. Jung, S. B. Han, J. T. Hong, Pharmacol. Ther. 2011, 130, 157–176.
- 20
- 20aP. Banerjee, A. Basu, J. L. Arbiser, S. Pal, Cancer Lett. 2013, 338, 292–299;
- 20bT. Singh, S. K. Katiyar, PLoS ONE 2013, 8, e 60749;
- 20cA. Kumar, U. Kumar Singh, A. Chaudhary, Future Med. Chem. 2013, 5, 809–829.
- 21C. W. Murray, D. C. Rees, Nat. Chem. 2009, 1, 187–192.
- 22D. A. Horton, G. T. Bourne, M. L. Smythe, Chem. Rev. 2003, 103, 893–930.
- 23A. Koppen, R. Houtman, D. Pijnenburg, E. H. Jeninga, R. Ruijtenbeek, E. Kalkhoven, Mol. Cell. Proteomics 2009, 8, 2212–2226.
- 24C. Abad-Zapatero, Expert Opin. Drug Discovery 2007, 2, 469–488.
- 25B. Y. Feng, B. K. Shoichet, Nat. Protoc. 2006, 1, 550–553.
- 26V. Nahoum, E. Pérez, P. Germain, F. Rodríguez-Barrios, F. Manzo, S. Kammerer, G. Lemaire, O. Hirsch, C. A. Royer, H. Gronemeyer, et al., Proc. Natl. Acad. Sci. USA 2007, 104, 17323–17328.
- 27K. L. Billingsley, T. E. Barder, S. L. Buchwald, Angew. Chem. 2007, 119, 5455–5459;
10.1002/ange.200701551 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5359–5363.