The Highly Lewis Acidic Dicationic Phosphonium Salt: [(SIMes)PFPh2][B(C6F5)4]2†
Dr. Michael H. Holthausen
Department of Chemistry, University of Toronto, 80 St. George St, Toronto Ontario M5S3H6 (Canada)
Search for more papers by this authorMeera Mehta
Department of Chemistry, University of Toronto, 80 St. George St, Toronto Ontario M5S3H6 (Canada)
Search for more papers by this authorCorresponding Author
Prof. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, 80 St. George St, Toronto Ontario M5S3H6 (Canada)
Chemistry Department-Faculty of Science, King Abdulaziz University, Jeddah 21589 (Saudi Arabia)
Department of Chemistry, University of Toronto, 80 St. George St, Toronto Ontario M5S3H6 (Canada)Search for more papers by this authorDr. Michael H. Holthausen
Department of Chemistry, University of Toronto, 80 St. George St, Toronto Ontario M5S3H6 (Canada)
Search for more papers by this authorMeera Mehta
Department of Chemistry, University of Toronto, 80 St. George St, Toronto Ontario M5S3H6 (Canada)
Search for more papers by this authorCorresponding Author
Prof. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, 80 St. George St, Toronto Ontario M5S3H6 (Canada)
Chemistry Department-Faculty of Science, King Abdulaziz University, Jeddah 21589 (Saudi Arabia)
Department of Chemistry, University of Toronto, 80 St. George St, Toronto Ontario M5S3H6 (Canada)Search for more papers by this authorD.W.S. gratefully acknowledges the financial support of the NSERC of Canada and the award of a Canada Research Chair. M.H.H. thanks the Alexander von Humboldt Foundation for a Feodor Lynen Research Fellowship.
Graphical Abstract
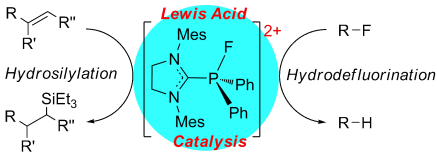
Versatile phosphonium salt: The dication [(SIMes)PFPh2][B(C6F5)4]2 is prepared by oxidation of an NHC-derived cationic phosphine followed by fluoride abstraction. This species exhibits remarkable Lewis acidity in stoichiometric reactions as well as in Lewis acid catalysis of hydrodefluorination of fluoroalkanes and the hydrosilylation of olefins and acetylenes.
Abstract
The dicationic imidazolium-phosphonium salt [(SIMes)PFPh2][B(C6F5)4]2 has been prepared and shown to exhibit remarkable Lewis acidity in stoichiometric reactions and acting as an effective Lewis acid catalyst for the hydrodefluorination of fluoroalkanes and the hydrosilylation of olefins.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403693_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. Burford, P. J. Ragogna, J. Chem. Soc. Dalton Trans. 2002, 4307–4315;
- 1bO. Guerret, G. Bertrand, Acc. Chem. Res. 1997, 30, 486–493;
- 1cD. Gudat, Acc. Chem. Res. 2010, 43, 1307–1316.
- 2J. M. Slattery, S. Hussein, Dalton Trans. 2012, 41, 1808–1815.
- 3A. H. Cowley, R. A. Kemp, Chem. Rev. 1985, 85, 367–382.
- 4
- 4aM. H. Holthausen, K. Feldmann, S. Schulz, A. Hepp, J. J. Weigand, Inorg. Chem. 2012, 51, 3374–3387;
- 4bM. H. Holthausen, A. Hepp, J. J. Weigand, Chem. Eur. J. 2013, 19, 9895–9907;
- 4cC. A. Dyker, N. Burford, Chem. Asian J. 2008, 3, 28–36.
- 5
- 5aT. W. Hudnall, Y. M. Kim, M. W. P. Bebbington, D. Bourissou, F. P. Gabbaï, J. Am. Chem. Soc. 2008, 130, 10890–10891;
- 5bC. W. Chiu, Y. Kim, F. P. Gabbai, J. Am. Chem. Soc. 2009, 131, 60–61.
- 6A. Werner, Adv. Synth. Catal. 2009, 351, 1469–1481.
- 7M. Terada, M. Kouchi, Tetrahedron 2006, 62, 401–409.
- 8G. Wittig, U. Schöllkopf, Chem. Ber. 1954, 87, 1318.
- 9
- 9aM. Pérez, L. J. Hounjet, C. B. Caputo, R. Dobrovetsky, D. W. Stephan, J. Am. Chem. Soc. 2013, 135, 18308–18310;
- 9bC. B. Caputo, L. J. Hounjet, R. Dobrovetsky, D. W. Stephan, Science 2013, 341, 1374–1377;
- 9cL. J. Hounjet, C. B. Caputo, D. W. Stephan, Dalton Trans. 2013, 42, 2629–2635.
- 10
- 10aO. Back, B. Donnadieu, P. Parameswaran, G. Frenking, G. Bertrand, Nat. Chem. 2010, 2, 369–373;
- 10bJ. Carreras, M. Patil, W. Thiel, M. Alcarazo, J. Am. Chem. Soc. 2012, 134, 16753–16758;
- 10cM. H. Holthausen, S. K. Surmiak, P. Jerabek, G. Frenking, J. J. Weigand, Angew. Chem. 2013, 125, 11284–11288; Angew. Chem. Int. Ed. 2013, 52, 11078–11082;
- 10dJ. Petuškova, M. Patil, S. Holle, C. W. Lehmann, W. Thiel, M. Alcarazo, J. Am. Chem. Soc. 2011, 133, 20758–20760.
- 11D. Mendoza-Espinosa, B. Donnadieu, G. Bertrand, J. Am. Chem. Soc. 2010, 132, 7264–7265.
- 12
- 12aT. Böttcher, O. Shyshkov, M. Bremer, B. S. Bassil, G.-V. Röschenthaler, Organometallics 2012, 31, 1278–1280;
- 12bK. Schwedtmann, M. H. Holthausen, K. O. Feldmann, J. J. Weigand, Angew. Chem. Int. Ed. 2013, 52, 14204–14208; Angew. Chem. 2013, 125, 14454–14458.
- 13C. Maaliki, C. Lepetit, C. Duhayon, Y. Canac, R. Chauvin, Chem. Eur. J. 2012, 18, 16153–16160.
- 14A. P. M. Robertson, N. Burford, R. McDonald, M. J. Ferguson, Angew. Chem. Int. Ed. 2014, 53, 3480–3483; Angew. Chem. 2014, 126, 3548–3551.
- 15
- 15aS. Conejero, M. Y. Song, D. Martin, Y. Canac, M. Soleilhavoup, G. Bertrand, Chem. Asian J. 2006, 1, 155–160;
- 15bS. M. Huber, F. W. Heinemann, P. Audebert, R. Weiss, Chem. Eur. J. 2011, 17, 13078–13086.
- 16V. Gutmann, Coord. Chem. Rev. 1976, 18, 225–255.
- 17R. F. Childs, D. L. Mulholland, A. Nixon, Can. J. Chem. 1982, 60, 801–808.
- 18
- 18aA. G. Evans, E. D. Owen, J. Chem. Soc. 1959, 4123–4125;
- 18bL. J. Hounjet, C. Bannwarth, C. N. Garon, C. B. Caputo, S. Grimme, D. W. Stephan, Angew. Chem. Int. Ed. 2013, 52, 7492–7495; Angew. Chem. 2013, 125, 7640–7643.
- 19
- 19aC. B. Caputo, D. W. Stephan, Organometallics 2012, 31, 27–30;
- 19bT. Stahl, H. Klare, M. Oestreich, ACS Catal. 2013, 3, 1578–1587.
- 20C. Douvris, O. V. Ozerov, Science 2008, 321, 1188–1190.
- 21M. Alcarazo, C. Gomez, S. Holle, R. Goddard, Angew. Chem. Int. Ed. 2010, 49, 5788–5791; Angew. Chem. 2010, 122, 50–81.
- 22W. Gu, M. R. Haneline, C. Douvris, O. V. Ozerov, J. Am. Chem. Soc. 2009, 131, 11203–11212.
- 23
- 23aS. Rendler, M. Oestreich, Angew. Chem. Int. Ed. 2008, 47, 5997–6000; Angew. Chem. 2008, 120, 6086–6089;
- 23bJ. Hermeke, M. Mewald, M. Oestreich, J. Am. Chem. Soc. 2013, 135, 17537–17546;
- 23cM. Rubin, T. Schwier, V. Gevorgyan, J. Org. Chem. 2002, 67, 1936–1940.