Synthesis of Tetrahydropyran/Tetrahydrofuran-Containing Macrolides by Palladium-Catalyzed Alkoxycarbonylative Macrolactonizations†
Yu Bai
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907 (USA) http://www.chem.purdue.edu/dai/
Search for more papers by this authorDexter C. Davis
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907 (USA) http://www.chem.purdue.edu/dai/
Search for more papers by this authorCorresponding Author
Prof. Dr. Mingji Dai
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907 (USA) http://www.chem.purdue.edu/dai/
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907 (USA) http://www.chem.purdue.edu/dai/Search for more papers by this authorYu Bai
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907 (USA) http://www.chem.purdue.edu/dai/
Search for more papers by this authorDexter C. Davis
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907 (USA) http://www.chem.purdue.edu/dai/
Search for more papers by this authorCorresponding Author
Prof. Dr. Mingji Dai
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907 (USA) http://www.chem.purdue.edu/dai/
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907 (USA) http://www.chem.purdue.edu/dai/Search for more papers by this authorWe thank Professor Yu Xia’s group at Purdue University for mass spectrometric assistance, the NYU Molecular Design Institute for the purchase of the Bruker SMART APEXII Diffractometer, and Dr. Chunhua Hu for his assistance with data collection and structure determination. Financial support from the Purdue University and Purdue Center for Cancer Research is gratefully acknowledged. We thank the NIH P30CA023168 for supporting shared resources to Purdue University Center for Cancer Research. Y.B. thanks the Purdue Research Foundation for a research assistantship and D.C.D. thanks the H. C. Brown Funds for a summer research support. M.D. is a recipient of the 2013 Showalter Research Trust Award and 2013 ORAU Ralph E. Powe Junior Faculty Enhancement Award.
Graphical Abstract
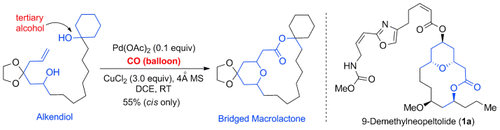
CO for bridged macrolides: An efficient Pd-catalyzed cascade alkoxycarbonylative macrolactonization to synthesize various THP/THF-containing macrolactones in one step from relatively simple alkendiols is possible. Challenging macrolactones involving tertiary alcohols were synthesized smoothly as well. The method was applied to the synthesis of the potent anticancer compound 9-demethylneopeltolide.
Abstract
A novel Pd-catalyzed cascade alkoxycarbonylative macrolactonization to construct tetrahydropyran/tetrahydrofuran-containing bridged macrolactones in one step from alkendiols is described. Products with various ring sizes and substituents were obtained. Challenging macrolactones involving tertiary alcohols were synthesized smoothly as well. Mechanistically, experimental evidence to support a trans-oxypalladation step has been provided. The method was applied to the synthesis of potent anticancer compound 9-demethylneopeltolide.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403006_sm_miscellaneous_information.pdf9.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. M. Driggers, S. P. Hale, J. Lee, N. K. Terrett, Nat. Rev. Drug Discovery 2008, 7, 608–624;
- 1bF. Kopp, C. F. Stratton, L. B. Akella, D. S. Tan, Nat. Chem. Biol. 2012, 8, 358–365.
- 2A. Lorente, J. Lamariano-Merketegi, F. Albericio, M. Álvarez, Chem. Rev. 2013, 113, 4567–4610.
- 3A. E. Wright, J. C. Botelho, E. Guzmán, D. Harmody, P. Linley, P. J. McCarthy, T. P. Pitts, S. A. Pomponi, J. K. Reed, J. Nat. Prod. 2007, 70, 412–416.
- 4H. Luesch, W. Y. Yoshida, G. G. Harrigan, J. P. Doom, R. E. Moore, V. J. Paul, J. Nat. Prod. 2002, 65, 1945–1948.
- 5
- 5aS. Ohta, M. M. Uy, M. Yanai, E. Ohta, T. Hirata, S. Ikegami, Tetrahedron Lett. 2006, 47, 1957–1960;
- 5bE. A. Crane, T. P. Zabawa, R. L. Farmer, K. A. Scheidt, Angew. Chem. 2011, 123, 9278–9281;
10.1002/ange.201102790 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9112–9115;
- 5cH. Fuwa, T. Suzuki, H. Kubo, T. Yamori, M. Sasaki, Chem. Eur. J. 2011, 17, 2678–2688.
- 6For reviews:
- 6aA. Parenty, X. Moreau, G. Niel, J.-M. Campagne, Chem. Rev. 2013, 113, PR 1–PR40;
- 6bA. Parenty, X. Moreau, J.-M. Campagne, Chem. Rev. 2006, 106, 911–939.
- 7
- 7aR. H. Grubbs, Handbook of Metathesis, Vol. 2, Wiley-VCH, Weinheim, 2003;
10.1002/9783527619481 Google Scholar
- 7bJ. Cossy, S. Arseniyadis, C. Meyer, Metathesis in Natural Product Synthesis, Wiley-VCH, Weinheim, 2010.
10.1002/9783527629626 Google Scholar
- 8
- 8aD. W. Custar, T. P. Zabawa, K. A. Scheidt, J. Am. Chem. Soc. 2008, 130, 804–805;
- 8bS. K. Woo, M. S. Kwon, E. Lee, Angew. Chem. 2008, 120, 3286–3288; Angew. Chem. Int. Ed. 2008, 47, 3242–3244;
- 8cP. A. Wender, B. A. DeChristopher, A. J. Schrier, J. Am. Chem. Soc. 2008, 130, 6658–6659;
- 8dK. B. Bahnck, S. D. Rychnovsky, J. Am. Chem. Soc. 2008, 130, 13177–13181.
- 9
- 9aT. R. Hoye, M. E. Danielson, A. E. May, H. Zhao, Angew. Chem. 2008, 120, 9889–9892;
10.1002/ange.200804049 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9743–9746;
- 9bT. R. Hoye, M. E. Danielson, A. E. May, H. Zhao, J. Org. Chem. 2010, 75, 7052–7060.
- 10
- 10aF. Hilli, J. M. White, M. A. Rizzacasa, Org. Lett. 2004, 6, 1289–1292;
- 10bM. Kanematsu, M. Yoshida, K. Shishido, Angew. Chem. 2011, 123, 2666–2668; Angew. Chem. Int. Ed. 2011, 50, 2618–2620.
- 11
- 11aM. F. Semmelhack, C. Bodurow, J. Am. Chem. Soc. 1984, 106, 1496–1498;
- 11bM. F. Semmelhack, C. Kim, N. Zhang, C. Bodurow, M. Sanner, W. Dobler, M. Meier, Pure Appl. Chem. 1990, 62, 2035–2040;
- 11cZ. Li, Y. Gao, Z. Jiao, N. Wu, D. Z. Wang, Z. Yang, Org. Lett. 2008, 10, 5163–5166. For examples of synthetic applications:
- 11dJ. Carpenter, A. B. Northrup, D. Chung, J. J. M. Wiener, S.-G. Kim, D. W. C. MacMillan, Angew. Chem. 2008, 120, 3624–3628;
10.1002/ange.200800086 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3568–3572;
- 11eZ. Yang, X. Xie, P, Jing, G. Zhao, J. Zheng, C. Zhao, X. She, Org. Biomol. Chem. 2011, 9, 984–986;
- 11fQ. Xiao, W.-W. Ren, Z.-X. Chen, T.-W. Sun, Y. Li, Q.-D. Ye, J.-X. Gong, F.-K. Meng, L. You, Y.-F. Liu, M.-Z. Zhao, L.-M. Xu, Z.-H. Shan, Y. Shi, Y.-F. Tang, J.-H. Chen, Z. Yang, Angew. Chem. 2011, 123, 7511–7515; Angew. Chem. Int. Ed. 2011, 50, 7373–7377.
- 12
- 12aX.-F. Wu, H. Neumann, M. Beller, Chem. Rev. 2013, 113, 1–35;
- 12bS.-M. Lu, H. Alper, J. Am. Chem. Soc. 2005, 127, 14776–14784;
- 12cS.-M. Lu, H. Alper, Chem. Eur. J. 2007, 13, 5908–5916;
- 12dS.-M. Lu, H. Alper, J. Am. Chem. Soc. 2008, 130, 6451–6455;
- 12eG. Lenoble, M. Urrutigoity, P. Kalck, Tetrahedron Lett. 2001, 42, 3697–3700;
- 12fJ. C. Knight, R. Prabaharan, B. D. Ward, A. J. Amoroso, P. G. Edwards, B. M. Kariuki, Dalton Trans. 2010, 39, 10031–10033;
- 12gM. Setoh, O. Yamada, K. Ogasawara, Heterocycles 1995, 40, 539–542;
- 12hE. Yoneda, S.-W. Zhang, K. Onitsuka, S. Takahashi, Tetrahedron Lett. 2001, 42, 5459–5461;
- 12iT. Takahashi, S.-i. Kusaka, T. Doi, T. Sunazuka, S. Omura, Angew. Chem. 2003, 115, 5388–5392; Angew. Chem. Int. Ed. 2003, 42, 5230–5234.
- 13
- 13aT. A. Cernak, T. H. Lambert, J. Am. Chem. Soc. 2009, 131, 3124–3125;
- 13bL. A. Ambrosini, T. A. Cernak, T. H. Lambert, Synthesis 2010, 870–881;
- 13cL. A. Ambrosini, T. A. Cernak, T. H. Lambert, Tetrahedron 2010, 66, 4882–4887.
- 14CCDC 985926 contains the supplementary crystallographic data for compound cis-7 m of this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15
- 15aJ. K. Stille, R. Divakaruni, J. Organomet. Chem. 1979, 169, 239–248. For examples of trans-oxypalladation in the Wacker reaction, see:
- 15bJ. E. Bäckvall, B. Akermark, S. O. Ljunggren, J. Am. Chem. Soc. 1979, 101, 2411–2416;
- 15cT. Majima, H. Kurosawa, J. Chem. Soc. Chem. Commun. 1977, 610–611.
- 16
- 16aR. M. Trend, Y. K. Ramtohul, B. M. Stoltz, J. Am. Chem. Soc. 2005, 127, 17778–17788;
- 16bY. Uozumi, K. Kato, T. Hayashi, J. Am. Chem. Soc. 1997, 119, 5063–5064;
- 16cT. Hayashi, K. Yamasaki, M. Mimura, Y. Uozumi, J. Am. Chem. Soc. 2004, 126, 3036–3037;
- 16dM. B. Hay, A. R. Hardin, J. P. Wolfe, J. Org. Chem. 2005, 70, 3099–3107;
- 16eJ. W. Francis, P. M. Henry, J. Mol. Catal. A 1996, 112, 317–326.
- 17
- 17aG. Liu, S. S. Stahl, J. Am. Chem. Soc. 2007, 129, 6328–6335;
- 17bJ. E. Bäckvall, Acc. Chem. Res. 1983, 16, 335–342.
- 18J. S. Quesnel, B. A. Arndtsen, J. Am. Chem. Soc. 2013, 135, 16841–16844.
- 19
- 19aS. Masamune, Y. Hayase, W. Schilling, W. K. Chan, G. S. Bates, J. Am. Chem. Soc. 1977, 99, 6756–6758;
- 19bP. M. Booth, C. M. Fox, S. V. Ley, J. Chem. Soc. Perkin Trans. 1 1987, 121–129;
- 19cD. S. Clyne, L. Weiler, Tetrahedron 1999, 55, 13659–13682;
- 19dA. ElMarrouni, R. Lebeuf, J. Gebauer, M. Heras, S. Arseniyadis, J. Cossy, Org. Lett. 2012, 14, 314–317.
- 20For the synthesis and biological evaluation of 9-demethylneopeltolide: H. Fuwa, A. Saito, S. Naito, K. Konoki, M. Yotsu-Yamashita, M. Sasaki, Chem. Eur. J. 2009, 15, 12807–12818.
- 21For total syntheses of neopeltolide: references [8a] and [8b], and
- 21aW. Youngsaye, J. T. Lowe, F. Pohlki, P. Ralifo, J. S. Panek, Angew. Chem. 2007, 119, 9371–9374;
10.1002/ange.200704122 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 9211–9214;
- 21bI. Paterson, N. A. Miller, Chem. Commun. 2008, 4708–4710;
- 21cH. Fuwa, S. Naito, T. Goto, M. Sasaki, Angew. Chem. 2008, 120, 4815–4817;
10.1002/ange.200801399 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4737–4739.
- 21dO. A. Ulanovskaya, J. Janjic, M. Suzuki, S. S. Sabharwal, P. T. Schumacker, S. J. Kron, S. A. Kozmin, Nat. Chem. Biol. 2008, 4, 418–424;
- 21eX. Guinchard, E. Roulland, Org. Lett. 2009, 11, 4700–4703;
- 21fH. Fuwa, A. Saito, M. Sasaki, Angew. Chem. 2010, 122, 3105–3108;
10.1002/ange.201000624 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3041–3044;
- 21gA. K. Ghosh, K. A. Shurrush, Z. L. Dawson, Org. Biomol. Chem. 2013, 11, 7768–7777. For formal synthesis:
- 21hV. V. Vintonyak, M. E. Maier, Org. Lett. 2008, 10, 1239–1242;
- 21iR. Kartika, T. R. Gruffi, R. E. Taylor, Org. Lett. 2008, 10, 5047–5050;
- 21jW. Tu, P. E. Floreancig, Angew. Chem. 2009, 121, 4637–4641; Angew. Chem. Int. Ed. 2009, 48, 4567–4571;
- 21kH. Kim, Y. Park, J. Hong, Angew. Chem. 2009, 121, 7713–7717; Angew. Chem. Int. Ed. 2009, 48, 7577–7581;
- 21lJ. S. Yadav, G. G. Krishana, S. N. Kumar, Tetrahedron 2010, 66, 480–487;
- 21mD. Martinez-Solorio, M. P. Jennings, J. Org. Chem. 2010, 75, 4095–4104;
- 21nZ. Yang, B. Zhang, G. Zhao, J. Yang, X. Xie, X. She, Org. Lett. 2011, 13, 5916–5919;
- 21oS. Raghavan, P. K. Samanta, Org. Lett. 2012, 14, 2346–2349;
- 21pG. V. M. Sharma, S. V. Reddy, K. V. S. Ramakrishna, Org. Biomol. Chem. 2012, 10, 3689–3695. For analog synthesis:
- 21qV. V. Vintonyak, B. Kunze, F. Sasse, M. E. Maier, Chem. Eur. J. 2008, 14, 11132–11140;
- 21rD. W. Custar, T. P. Zabawa, J. Hines, C. M. Crews, K. A. Scheidt, J. Am. Chem. Soc. 2009, 131, 12406–12414;
- 21sY. Cui, W. Tu, P. E. Floreancig, Tetrahedron 2010, 66, 4867–4873;
- 21tY. Cui, R. Balachandran, B. W. Day, P. E. Floreancig, J. Org. Chem. 2012, 77, 2225–2235;
- 21uH. Fuwa, M. Kawakami, K. Noto, T. Muto, Y. Suga, K. Konoki, M. Yotsu-Yamashita, M. Sasaki, Chem. Eur. J. 2013, 19, 8100–8110.
- 22Y. Wang, J. Janjic, S. A. Kozmin, J. Am. Chem. Soc. 2002, 124, 13670–13671.