Palladium-Catalyzed Oxidative Difunctionalization of Alkenes with α-Carbonyl Alkyl Bromides Initiated through a Heck-type Insertion: A Route to Indolin-2-ones†
Jian-Hong Fan
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorWen-Ting Wei
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorMing-Bo Zhou
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorDr. Ren-Jie Song
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Heng Li
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000 (China)
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)Search for more papers by this authorJian-Hong Fan
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorWen-Ting Wei
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorMing-Bo Zhou
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorDr. Ren-Jie Song
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Heng Li
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000 (China)
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082 (China)Search for more papers by this authorWe thank the Natural Science Foundation of China (21172060), the Specialized Research Fund for the Doctoral Program of Higher Education (20120161110041), and the Hunan Provincial Natural Science Foundation of China (13JJ2018) for financial support.
Graphical Abstract
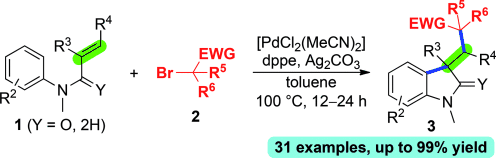
Indolinone synthesis: A new palladium-catalyzed oxidative difunctionalization reaction of N-arylalkenes with primary, secondary, and tertiary α-carbonyl alkyl bromides proceeds through a radical process and provides indolin-2-ones in moderate to excellent yields. The reaction is initiated by a Heck insertion followed by interception of the σ-alkyl palladium(II) intermediate with aryl C(sp2)H bonds. dppe=1,2-bis(diphenylphosphino)ethane.
Abstract
The oxidative interception of various σ-alkyl palladium(II) intermediates with additional reagents for the difunctionalization of alkenes is an important research area. A new palladium-catalyzed oxidative difunctionalization reaction of alkenes with α-carbonyl alkyl bromides is described, in which the σ-alkyl palladium(II) intermediate is generated through a Heck insertion and trapped using an aryl C(sp2)H bond. This method can be applied to various α-carbonyl alkyl bromides, including primary, secondary, and tertiary α-bromoalkyl esters, ketones, and amides.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402893_sm_miscellaneous_information.pdf5.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. F. Heck, J. P. Nolley, J. Org. Chem. 1972, 37, 2320;
- 1bT. Mizoroki, K. Mori, A. Ozaki, Bull. Chem. Soc. Jpn. 1971, 44, 581; for selected reviews, see:
- 1cA. C. Frisch, M. Beller, Angew. Chem. 2005, 117, 680; Angew. Chem. Int. Ed. 2005, 44, 674;
- 1dM. Oestreich, The Mizoroki-Heck reaction, Wiley, Hoboken, NJ, 2008;
- 1eA. Rudolph, M. Lautens, Angew. Chem. 2009, 121, 2694;
10.1002/ange.200803611 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2656;
- 1fB. Karimi, H. Behzadnia, D. Elhamifar, P. E. Akhavan, F. K. Esfahani, A. Zamani, Synthesis 2010, 1399;
- 1gD. Mc Cartney, P. J. Guiry, Chem. Soc. Rev. 2011, 40, 5122;
- 1hB. W. Glasspoole, C. M. Crudden, Nat. Chem. 2011, 3, 912.
- 2
- 2aM. Mori, I. Oda, Y. Ban, Tetrahedron Lett. 1982, 23, 5315;
- 2bG. Z. Wu, F. Lamaty, E. Negishi, J. Org. Chem. 1989, 54, 2507;
- 2cY. Pan, Z. Zhang, H. Hu, Synth. Commun. 1992, 22, 2019;
- 2dY. Pan, Z. Zhang, H. Hu, Synthesis 1995, 245;
- 2eP. Kumar, Org. Prep. Proced. Int. 1997, 29, 477;
- 2fL. Wang, Y. Pan, X. Jiang, H. Hu, Tetrahedron Lett. 2000, 41, 725;
- 2gF. Glorius, Tetrahedron Lett. 2003, 44, 5751;
- 2hK. Higuchi, K. Sawada, H. Nambu, T. Shogaki, Y. Kita, Org. Lett. 2003, 5, 3703;
- 2iH. Narahashi, A. Yamamoto, I. Shimizu, Chem. Lett. 2004, 33, 348;
- 2jJ. Terao, N. Kambe, Bull. Chem. Soc. Jpn. 2006, 79, 663;
- 2kL. Firmansjah, G. C. Fu, J. Am. Chem. Soc. 2007, 129, 11340;
- 2lS. Bräse, B. Waegell, M. A. De, Synthesis 1998, 148;
- 2mK. S. Bloome, E. J. Alexanian, J. Am. Chem. Soc. 2010, 132, 12823;
- 2nW. Zhou, G. An, G. Zhang, J. Han, Y. Pan, Org. Biomol. Chem. 2011, 9, 5833;
- 2oK. S. Bloome, R. L. McMahen, E. J. Alexanian, J. Am. Chem. Soc. 2011, 133, 20146;
- 2pZ. Yang, J. Zhou, J. Am. Chem. Soc. 2012, 134, 11833.
- 3
- 3aY. Ikeda, T. Nakamura, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc. 2002, 124, 6514;
- 3bT. Fujioka, T. Nakamura, H. Yorimitsu, K. Oshima, Org. Lett. 2002, 4, 2257;
- 3cY. Ikeda, H. Yorimitsu, H. Shinokubo, K. Oshima, Adv. Synth. Catal. 2004, 346, 1631;
- 3dW. Affo, H. Ohmiya, T. Fujioka, Y. Ikeda, T. Nakamura, H. Yorimitsu, K. Oshima, Y. Imamura, T. Mizuta, K. Miyoshi, J. Am. Chem. Soc. 2006, 128, 8068;
- 3eM. E. Weiss, L. M. Kreis, A. Lauber, E. M. Carreira, Angew. Chem. 2011, 123, 11321;
10.1002/ange.201105235 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11125.
- 4
- 4aS. A. Lebedev, V. S. Lopatina, E. S. Petrov, I. P. Beletskaya, J. Organomet. Chem. 1988, 344, 253;
- 4bR. Matsubara, A. C. Gutierrez, T. F. Jamison, J. Am. Chem. Soc. 2011, 133, 19020;
- 4cA. Millán, L. Álvarez de Cienfuegos, D. Miguel, A. G. Campaña, J. M. Cuerva, Org. Lett. 2012, 14, 5984;
- 4dC. Liu, S. Tang, D. Liu, J. Yuan, L. Zheng, L. Meng, A. Lei, Angew. Chem. 2012, 124, 3698; Angew. Chem. Int. Ed. 2012, 51, 3638;
- 4eA. Nakatani, K. Hirano, T. Satoh, M. Miura, Chem. Eur. J. 2013, 19, 7691;
- 4fE. A. Standley, T. F. Jamison, J. Am. Chem. Soc. 2013, 135, 1585.
- 5For examples of such processes that are catalyzed by other transition metals, see: ruthenium:
- 5aD.-H. Lee, K.-H. Kwon, C. S. Yi, Science 2011, 333, 1613; ruthenium or iridium:
- 5bQ. Liu, H. Yi, J. Liu, Y. Yang, X. Zhang, Z. Zeng, A. Lei, Chem. Eur. J. 2013, 19, 5120; copper:
- 5cT. Nishikata, Y. Noda, R. Fujimoto, T. Sakashita, J. Am. Chem. Soc. 2013, 135, 16372.
- 6
- 6aT. Kobayashi, H. Ohmiya, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc. 2008, 130, 11276;
- 6bA. C. Bissember, A. Levina, G. C. Fu, J. Am. Chem. Soc. 2012, 134, 14232.
- 7For selected examples, see: CO:
- 7aT. Sugihara, C. Coperet, Z. Owczarczyk, L. S. Harring, E.-i. Negishi, J. Am. Chem. Soc. 1994, 116, 7923;
- 7bG. D. Artman III, S. M. Weinreb, Org. Lett. 2003, 5, 1523; alkyne and alkene:
- 7cS. Schweizer, Z.-Z. Song, F. E. Meyer, P. J. Parsons, A. de Meijere, Angew. Chem. 1999, 111, 1550;
10.1002/(SICI)1521-3757(19990517)111:10<1550::AID-ANGE1550>3.0.CO;2-9 Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1452;10.1002/(SICI)1521-3773(19990517)38:10<1452::AID-ANIE1452>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 7dL. F. Tietze, K. Kahle, T. Raschke, Chem. Eur. J. 2002, 8, 401; ArB(OH)2:
10.1002/1521-3765(20020118)8:2<401::AID-CHEM401>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 7eL. Liao, R. Jana, K. B. Urkalan, M. S. Sigman, J. Am. Chem. Soc. 2011, 133, 5784;
- 7fV. Saini, M. S. Sigman, J. Am. Chem. Soc. 2012, 134, 11372;
- 7gV. Saini, L. Liao, Q. Wang, R. Jana, M. S. Sigman, Org. Lett. 2013, 15, 5008;
- 7hM. S. McCammant, L. Liao, M. S. Sigman, J. Am. Chem. Soc. 2013, 135, 4167; for selected reviews, see:
- 7iK. C. Nicolaou, D. J. Edmonds, P. G. Bulger, Angew. Chem. 2006, 118, 7292;
10.1002/ange.200601872 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7134;
- 7jS. R. Chemler, P. H. Fuller, Chem. Soc. Rev. 2007, 36, 1153;
- 7kJ. P. Wolfe, Synlett 2008, 2913.
- 8
- 8aR. F. Heck, J. Am. Chem. Soc. 1968, 90, 5538;
- 8bD. Kalyani, M. S. Sanford, J. Am. Chem. Soc. 2008, 130, 2150;
- 8cD. Kalyani, A. D. Satterfield, M. S. Sanford, J. Am. Chem. Soc. 2010, 132, 8419;
- 8dY. Tamaru, M. Hojo, H. Higashimura, Z. Yoshida, Angew. Chem. 1986, 98, 740; Angew. Chem. Int. Ed. Engl. 1986, 25, 735;
- 8eA. Rodriguez, W. J. Moran, Eur. J. Org. Chem. 2009, 1313;
- 8fA. D. Satterfield, A. Kubota, M. S. Sanford, Org. Lett. 2011, 13, 1076;
- 8gC. Zhu, J. R. Falck, Angew. Chem. 2011, 123, 6756; Angew. Chem. Int. Ed. 2011, 50, 6626;
- 8hY. Tamaru, M. Hojo, S. Kawamura, Z. Yoshida, J. Org. Chem. 1986, 51, 4089;
- 8iK. B. Urkalan, M. S. Sigman, Angew. Chem. 2009, 121, 3192;
10.1002/ange.200900218 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3146;
- 8jE. W. Werner, K. B. Urkalan, M. S. Sigman, Org. Lett. 2010, 12, 2848.
- 9
- 9aB. S. Jensen, CNS Drug Rev. 2002, 8, 353;
- 9bC. Marti, E. M. Carreira, Eur. J. Org. Chem. 2003, 2209;
- 9cH. Lin, S. J. Danishefsky, Angew. Chem. 2003, 115, 38; Angew. Chem. Int. Ed. 2003, 42, 36;
- 9dC. V. Galliford, K. A. Scheidt, Angew. Chem. 2007, 119, 8902;
10.1002/ange.200701342 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8748;
- 9eA. Millemaggi, R. J. K. Taylor, Eur. J. Org. Chem. 2010, 4527;
- 9fF. Zhou, Y.-L. Liu, J. Zhou, Adv. Synth. Catal. 2010, 352, 1381;
- 9gS. R. S. Rudrangi, V. K. Bontha, V. R. Manda, S. Bethi, Asian J. Res. Chem. 2011, 4, 335;
- 9hG. Deak, M. Doda, L. Gyorgy, L. Hazai, L. Sterk, J. Med. Chem. 1977, 20, 1384;
- 9iA. Numata, P. Yang, C. Takahashi, R. Fujiki, M. Nabae, E. Fujita, Chem. Pharm. Bull. 1989, 37, 648;
- 9jS. Hibino, T. Choshi, Nat. Prod. Rep. 2001, 18, 66;
- 9kA. S. Ratnayake, W. Y. Yoshida, S. L. Mooberry, T. K. Hemscheidt, J. Org. Chem. 2001, 66, 8717;
- 9lB. M. Trost, J. Xie, J. D. Sieber, J. Am. Chem. Soc. 2011, 133, 20611.
- 10For selected reviews on the use of Ag salts in organic synthesis, see:
- 10aM. Naodovic, H. Yamamoto, Chem. Rev. 2008, 108, 3132;
- 10bJ.-M. Weibel, A. Blanc, P. Pale, Chem. Rev. 2008, 108, 3149;
- 10cM. Álvarez-Corral, M. Muñoz-Dorado, I. Rodríguez-García, Chem. Rev. 2008, 108, 3174;
- 10d Silver in Organic Chemistry (Ed.: ), Wiley, Hoboken, NJ, 2010.
- 11
- 11aX. Wu, Y. Zhao, H. Ge, J. Am. Chem. Soc. 2014, 136, 1789; generally, PdII/AgI-catalyzed oxidative reactions proceed through a PdII/Pd0 process; see:
- 11bD. R. Stuart, E. Villemure, K. Fagnou, J. Am. Chem. Soc. 2007, 129, 12072;
- 11cZ. Liang, J. Zhao, Y. Zhang, J. Org. Chem. 2010, 75, 170;
- 11dM. Miyasaka, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2010, 75, 5421;
- 11eA. Ashimori, B. Bachand, L. E. Overman, D. J. Poon, J. Am. Chem. Soc. 1998, 120, 6477;
- 11fC. Li, T. Yano, N. Ishida, M. Murakami, Angew. Chem. 2013, 125, 9983; Angew. Chem. Int. Ed. 2013, 52, 9801;
- 11gS. Islam, I. Larrosa, Chem. Eur. J. 2013, 19, 15093;
- 11hE. J. Choi, E. Kim, Y. Lee, A. Jo, S. B. Park, Angew. Chem. 2014, 126, 1370; Angew. Chem. Int. Ed. 2014, 53, 1346.