Superoxide Formation on Isolated Cationic Gold Clusters†
We gratefully acknowledge the support of the Stichting voor Fundamenteel Onderzoek der Materie (FOM) for providing beam time on FELIX, and the FELIX staff for their skilful assistance, in particular Dr. B. Redlich and Dr. A. F. G. van der Meer. This work is supported by the Fritz-Haber Institute of the Max-Planck Society, the Cluster of Excellence “Unifying Concepts in Catalysis” coordinated by the Technical University Berlin and funded by the Deutsche Forschungsgemeinschaft (DFG), and through the DFG within the research unit FOR 1282 (FI 893/4). We thank Dr. C. Kerpal for implementation of the modified basin hopping algorithm.
Graphical Abstract
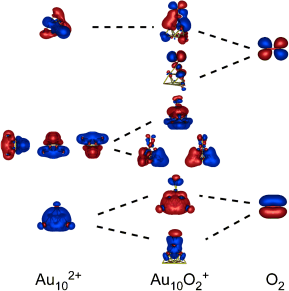
Act(ivat)ing positively: Cationic gold clusters are found to react with molecular oxygen and activate it by forming a superoxide (O2−), as confirmed by vibrational spectroscopy. This process is spontaneous in clusters, which attain a closed shell within the spherical jellium model, whereas other cluster sizes exhibit a self-promoting effect whereby the presence of multiple oxygen ligands is required for activation.
Abstract
Gold nanoparticles are known to be highly versatile oxidation catalysts utilizing molecular oxygen as a feedstock, but the mechanism and species responsible for activating oxygen remain unclear. The reaction between unsupported cationic gold clusters and molecular oxygen has been investigated. The resulting complexes were characterized in the gas phase using IR spectroscopy. A strong red-shift in the observed ν(O-O) stretching frequency indicates the formation of superoxo (O2−) moieties. These moieties are seen to form spontaneously in systems, which upon electron transfer attain a closed shell within the spherical jellium model (Au10+ and Au22+), whereas an oxygen induced self-promotion in the activation is observed for other systems (Au4+, Au12+, Au21+).