Mild Copper-Catalyzed Fluorination of Alkyl Triflates with Potassium Fluoride†
Hester Dang
Department of Chemistry, University of Washington, Seattle, WA 98195 (USA)
Search for more papers by this authorMelrose Mailig
Department of Chemistry, University of Washington, Seattle, WA 98195 (USA)
Search for more papers by this authorCorresponding Author
Prof. Gojko Lalic
Department of Chemistry, University of Washington, Seattle, WA 98195 (USA)
Department of Chemistry, University of Washington, Seattle, WA 98195 (USA)Search for more papers by this authorHester Dang
Department of Chemistry, University of Washington, Seattle, WA 98195 (USA)
Search for more papers by this authorMelrose Mailig
Department of Chemistry, University of Washington, Seattle, WA 98195 (USA)
Search for more papers by this authorCorresponding Author
Prof. Gojko Lalic
Department of Chemistry, University of Washington, Seattle, WA 98195 (USA)
Department of Chemistry, University of Washington, Seattle, WA 98195 (USA)Search for more papers by this authorWe thank the Royalty Research Fund at University of Washington and NSF (CAREER award no 1254636) for financial support. Prof. Forrest Michael and Prof. Dustin Maly are gratefully acknowledged for helpful discussions and suggestions.
Graphical Abstract
Teacher′s PET: The title reaction delivers excellent yields of the desired alkyl fluorides by using potassium fluoride as a fluoride source in the presence of the copper catalyst [IPrCuOTf]. This procedure is potentially suited for the preparation of 18F-labeled PET probes. IPr=1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, Tf=trifluoromethanesulfonyl.
Abstract
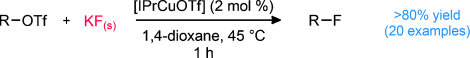
A chemoselective catalytic fluorination of alkyl triflates is described using potassium fluoride as a fluoride source. Excellent yields of the desired alkyl fluorides are obtained after one hour at 45 °C using 2 mol % of the copper catalyst. With 10 mol % of the catalyst, full conversion can be achieved in less than 10 minutes at 45 °C, and thus makes this procedure potentially suited for the preparation of 18F-labeled PET probes. As a result of the mild reaction conditions, only the substitution products are observed with no evidence of common side reactions, such as elimination. Reported is a preliminary study of the reaction scope, which demonstrates that the fluorination can be performed in the presence of a wide range of functional groups. Evidence suggests an unusual role of the [IPrCuOTf] catalyst as a phase-transfer catalyst and points to [IPrCuF] as the active fluorinating reagent (IPr=1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402238_sm_miscellaneous_information.pdf7.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 1bK. L. Kirk, Curr. Top. Med. Chem. 2006, 6, 1447–1456.
- 2
- 2aM. E. Phelps, Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233;
- 2bS. M. Ametamey, M. Honer, P. A. Schubiger, Chem. Rev. 2008, 108, 1501–1516.
- 3
- 3aZ. Jin, G. B. Hammond, B. Xu, Aldrichimica Acta 2012, 45, 67–83;
- 3bM. Tredwell, V. Gouverneur, Angew. Chem. 2012, 124, 11590–11602;
10.1002/ange.201204687 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11426–11437;
- 3cT. Furuya, J. E. M. N. Klein, T. Ritter, Synthesis 2010, 1804–1821.
- 4
- 4aD. A. Watson, M. Su, G. Teverovskiy, Y. Zhang, J. Garcia-Fortanet, T. Kinzel, S. L. Buchwald, Science 2009, 325, 1661–1664;
- 4bK. L. Hull, W. Q. Anani, M. S. Sanford, J. Am. Chem. Soc. 2006, 128, 7134–7135;
- 4cX. Wang, T.-S. Mei, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 7520–7521;
- 4dT. Furuya, H. M. Kaiser, T. Ritter, Angew. Chem. 2008, 120, 6082–6085;
10.1002/ange.200802164 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5993–5996;
- 4eT. Furuya, A. E. Strom, T. Ritter, J. Am. Chem. Soc. 2009, 131, 1662–1663;
- 4fP. Tang, T. Furuya, T. Ritter, J. Am. Chem. Soc. 2010, 132, 12150–12154;
- 4gM. Huiban, M. Tredwell, S. Mizuta, Z. Wan, X. Zhang, T. L. Collier, V. Gouverneur, J. Passchier, Nat. Chem. 2013, 5, 941–944;
- 4hE. Lee, A. S. Kamlet, D. C. Powers, C. N. Neumann, G. B. Boursalian, T. Furuya, D. C. Choi, J. M. Hooker, T. Ritter, Science 2011, 334, 639–642.
- 5
- 5aE. Benedetto, M. Tredwell, C. Hollingworth, T. Khotavivattana, J. M. Brown, V. Gouverneur, Chem. Sci. 2013, 4, 89–96;
- 5bM.-G. Braun, M. H. Katcher, A. G. Doyle, Chem. Sci. 2013, 4, 1216–1220;
- 5cJ. Zhu, G. C. Tsui, M. Lautens, Angew. Chem. 2012, 124, 12519–12522; Angew. Chem. Int. Ed. 2012, 51, 12353–12356;
- 5dC. Hollingworth, A. Hazari, M. N. Hopkinson, M. Tredwell, E. Benedetto, M. Huiban, A. D. Gee, J. M. Brown, V. Gouverneur, Angew. Chem. 2011, 123, 2661–2665; Angew. Chem. Int. Ed. 2011, 50, 2613–2617;
- 5eM. H. Katcher, A. Sha, A. G. Doyle, J. Am. Chem. Soc. 2011, 133, 15902–15905;
- 5fM.-G. Braun, A. G. Doyle, J. Am. Chem. Soc. 2013, 135, 12990–12993;
- 5gM. H. Katcher, A. G. Doyle, J. Am. Chem. Soc. 2010, 132, 17402–17404;
- 5hJ. J. Topczewski, T. J. Tewson, H. M. Nguyen, J. Am. Chem. Soc. 2011, 133, 19318–19321;
- 5iJ. M. Larsson, S. R. Pathipati, K. J. Szabó, J. Org. Chem. 2013, 78, 7330–7336;
- 5jA. M. Lauer, J. Wu, Org. Lett. 2012, 14, 5138–5141;
- 5kZ. Zhang, F. Wang, X. Mu, P. Chen, G. Liu, Angew. Chem. 2013, 125, 7697–7701; Angew. Chem. Int. Ed. 2013, 52, 7549–7553.
- 6C. Hollingworth, V. Gouverneur, Chem. Commun. 2012, 48, 2929–2942.
- 7
- 7aJ. A. Kalow, A. G. Doyle, J. Am. Chem. Soc. 2010, 132, 3268–3269;
- 7bJ. A. Kalow, D. E. Schmitt, A. G. Doyle, J. Org. Chem. 2012, 77, 4177–4183;
- 7cJ. A. Kalow, A. G. Doyle, J. Am. Chem. Soc. 2011, 133, 16001–16012;
- 7dT. J. Barker, D. L. Boger, J. Am. Chem. Soc. 2012, 134, 13588–13591;
- 7eF. Yin, Z. Wang, Z. Li, C. Li, J. Am. Chem. Soc. 2012, 134, 10401–10404;
- 7fW. Liu, X. Huang, M.-J. Cheng, R. J. Nielsen, W. A. Goddard, J. T. Groves, Science 2012, 337, 1322–1325;
- 7gC. Zhang, Z. Li, L. Zhu, L. Yu, Z. Wang, C. Li, J. Am. Chem. Soc. 2013, 135, 14082–14085;
- 7hS. Bloom, C. R. Pitts, D. C. Miller, N. Haselton, M. G. Holl, E. Urheim, T. Lectka, Angew. Chem. 2012, 124, 10732–10735;
10.1002/ange.201203642 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10580–10583;
- 7iM. Rueda-Becerril, C. Chatalova Sazepin, J. C. T. Leung, T. Okbinoglu, P. Kennepohl, J.-F. Paquin, G. M. Sammis, J. Am. Chem. Soc. 2012, 134, 4026–4029;
- 7kJ. C. T. Leung, C. Chatalova-Sazepin, J. G. West, M. Rueda-Becerril, J.-F. Paquin, G. M. Sammis, Angew. Chem. 2012, 124, 10962–10965; Angew. Chem. Int. Ed. 2012, 51, 10804–10807.
- 8D. W. Kim, D.-S. Ahn, Y.-H. Oh, S. Lee, H. S. Kil, S. J. Oh, S. J. Lee, J. S. Kim, J. S. Ryu, D. H. Moon, D. Y. Chi, J. Am. Chem. Soc. 2006, 128, 16394–16397.
- 9D. W. Kim, C. E. Song, D. Y. Chi, J. Am. Chem. Soc. 2002, 124, 10278–10279.
- 10K. Hamacher, H. H. Coenen, G. Stöcklin, J. Nucl. Med. 1986, 27, 235–238.
- 11C. L. Liotta, H. P. Harris, J. Am. Chem. Soc. 1974, 96, 2250–2252.
- 12S. Dermeik, Y. Sasson, J. Org. Chem. 1985, 50, 879–882.
- 13
- 13aP. W. Miller, N. J. Long, R. Vilar, A. D. Gee, Angew. Chem. 2008, 120, 9136–9172;
10.1002/ange.200800222 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8998–9033;
- 13bH. H. Coenen, M. Schüller, G. Stöcklin, B. Klatte, A. Knöchel, J. Labelled Compd. Radiopharm. 1986, 23, 455–466.
- 14J. H. Clark, Chem. Rev. 1980, 80, 429–452.
- 15J. Mukherjee, Z.-Y. Yang, M. K. Das, T. Brown, Nucl. Med. Biol. 1995, 22, 283–296.
- 16
- 16aJ. E. Veltheer, P. Burger, R. G. Bergman, J. Am. Chem. Soc. 1995, 117, 12478–12488;
- 16bB. K. Bennett, R. G. Harrison, T. G. Richmond, J. Am. Chem. Soc. 1994, 116, 11165–11166;
- 16cD. Breyer, T. Braun, P. Kläring, Organometallics 2012, 31, 1417–1424.
- 17Y. Liu, C. Chen, H. Li, K.-W. Huang, J. Tan, Z. Weng, Organometallics 2013, 32, 6587–6592.
- 18
- 18aJ. R. Herron, Z. T. Ball, J. Am. Chem. Soc. 2008, 130, 16486–16487;
- 18bH. Dang, N. Cox, G. Lalic, Angew. Chem. Int. Ed. 2014, 53, 752–756.
- 19K. Chen, P. S. Conti, Adv. Drug Delivery Rev. 2010, 62, 1005–1022.
- 20L. Li, M. N. Hopkinson, R. L. Yona, R. Bejot, A. D. Gee, V. Gouverneur, Chem. Sci. 2011, 2, 123–131.
- 21T. R. DeGrado, S. W. Baldwin, S. Wang, M. D. Orr, R. P. Liao, H. S. Friedman, R. Reiman, D. T. Price, R. E. Coleman, J. Nucl. Med. 2001, 42, 1805–1814.
- 22A. T. I. Jensen, T. Binderup, T. L. Andresen, A. Kjær, P. H. Rasmussen, J. Liposome Res. 2012, 22, 295–305.
- 23H. Li, J.-l. Zhou, X.-s. Chen, Russ. J. Phys. Chem. 2012, 86, 1940–1942.
- 24See the Supporting Information for details of stereochemistry assignment. Attempts to prepare enantioenriched version of the product 18 did not allow us to rigorously establish the stereochemistry of the fluorination reaction.
- 25See the Supporting Information for details.