Carbonylations of Alkenes with CO Surrogates
Lipeng Wu
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/
Search for more papers by this authorDr. Qiang Liu
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/
Search for more papers by this authorDr. Ralf Jackstell
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Beller
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/Search for more papers by this authorLipeng Wu
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/
Search for more papers by this authorDr. Qiang Liu
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/
Search for more papers by this authorDr. Ralf Jackstell
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Beller
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/
Leibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany) http://www.catalysis.de/Search for more papers by this authorGraphical Abstract
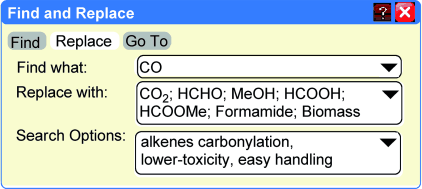
All current industrial carbonylation processes rely on highly toxic and flammable carbon monoxide. Since these properties impede the wider use of carbonylation reactions in industry and academia, performing carbonylations with CO surrogates is highly desired and will contribute to further advances in sustainable chemistry. This Minireview summarizes the carbonylations of alkenes using different CO surrogates.
Abstract
Alkene carbonylation reactions are important for the production of value-added bulk and fine chemicals. Nowadays, all industrial carbonylation processes make use of highly toxic and flammable carbon monoxide. In fact, these properties impede the wider use of carbonylation reactions in industry and academia. Hence, performing carbonylations without the use of CO is highly desired and will contribute to the further advancement of sustainable chemistry. Although the use of carbon monoxide surrogates in alkene carbonylation reactions has been reported intermittently in the last 30 years, only recently has this area attracted significant interest. This Minireview summarizes carbonylation reactions of alkenes using different carbon monoxide surrogates.
References
- 1
- 1aC. Jimenez Rodriguez, D. F. Foster, G. R. Eastham, D. J. Cole-Hamilton, Chem. Commun. 2004, 1720–1721;
- 1bI. del Río, C. Claver, P. W. N. M. van Leeuwen, Eur. J. Inorg. Chem. 2001, 2719–2738.
- 2
- 2aE. Zuidema, L. Escorihuela, T. Eichelsheim, J. J. Carbo, C. Bo, P. C. Kamer, P. W. van Leeuwen, Chem. Eur. J. 2008, 14, 1843–1853;
- 2bO. Diebolt, C. Muller, D. Vogt, Catal. Sci. Technol. 2012, 2, 773–777.
- 3T. Morimoto, K. Kakiuchi, Angew. Chem. 2004, 116, 5698–5706;
10.1002/ange.200301736 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5580–5588.
- 4For selected recent examples using CO surrogates in organic synthesis:
- 4aB. Yu, Y. Zhao, H. Zhang, J. Xu, L. Hao, X. Gao, Z. Liu, Chem. Commun. 2014, 50, 2330–2333;
- 4bS. Korsager, D. U. Nielsen, R. H. Taaning, T. Skrydstrup, Angew. Chem. 2013, 125, 9945–9948;
10.1002/ange.201304072 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 9763–9766;
- 4cT. Ueda, H. Konishi, K. Manabe, Angew. Chem. 2013, 125, 8773–8777; Angew. Chem. Int. Ed. 2013, 52, 8611–8615;
- 4dS. R. Borhade, A. Sandström, P. I. Arvidsson, Org. Lett. 2013, 15, 1056–1059;
- 4eD. N. Sawant, Y. S. Wagh, K. D. Bhatte, B. M. Bhanage, J. Org. Chem. 2011, 76, 5489–5494;
- 4fY. Katafuchi, T. Fujihara, T. Iwai, J. Terao, Y. Tsuji, Adv. Synth. Catal. 2011, 353, 475–482;
- 4gP. Hermange, A. T. Lindhardt, R. H. Taaning, K. Bjerglund, D. Lupp, T. Skrydstrup, J. Am. Chem. Soc. 2011, 133, 6061–6071;
- 4hJ. H. Park, Y. Cho, Y. K. Chung, Angew. Chem. 2010, 122, 5264–5267; Angew. Chem. Int. Ed. 2010, 49, 5138–5141;
- 4iO. Lagerlund, M. L. H. Mantel, M. Larhed, Tetrahedron 2009, 65, 7646–7652;
- 4jH. W. Lee, A. S. C. Chan, F. Y. Kwong, Chem. Commun. 2007, 2633–2635.
- 5
- 5aK. Tsuchiya, J.-D. Huang, K.-I. Tominaga, ACS Catal. 2013, 3, 2865–2868;
- 5bK.-i. Tominaga, Catal. Today 2006, 115, 70–72.
- 6E.-A. Jo, J.-H. Lee, C.-H. Jun, Chem. Commun. 2008, 0, 5779–5781.
- 7J. J. Verendel, M. Nordlund, P. G. Andersson, ChemSusChem 2013, 6, 426–429.
- 8T. Sakakura, J.-C. Choi, H. Yasuda, Chem. Rev. 2007, 107, 2365–2387.
- 9
- 9aS. Wesselbaum, T. Vom Stein, J. Klankermayer, W. Leitner, Angew. Chem. 2012, 124, 7617–7620;
10.1002/ange.201202320 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 7499–7502;
- 9bC. A. Huff, M. S. Sanford, J. Am. Chem. Soc. 2011, 133, 18122–18125;
- 9cE. Balaraman, C. Gunanathan, J. Zhang, L. J. W. Shimon, D. Milstein, Nat. Chem. 2011, 3, 609–614;
- 9dZ. Han, L. Rong, J. Wu, L. Zhang, Z. Wang, K. Ding, Angew. Chem. 2012, 124, 13218–13222; Angew. Chem. Int. Ed. 2012, 51, 13041–13045.
- 10M. Peters, B. Köhler, W. Kuckshinrichs, W. Leitner, P. Markewitz, T. E. Müller, ChemSusChem 2011, 4, 1216–1240.
- 11K. Nakano, K. Kobayashi, T. Ohkawara, H. Imoto, K. Nozaki, J. Am. Chem. Soc. 2013, 135, 8456–8459.
- 12
- 12aP. G. Jessop, T. Ikariya, R. Noyori, Chem. Rev. 1995, 95, 259–272;
- 12bC. Federsel, R. Jackstell, M. Beller, Angew. Chem. 2010, 122, 6392–6395;
10.1002/ange.201000533 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6254–6257.
- 13P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert, C. L. Liotta, Nature 2005, 436, 1102–1102.
- 14K. Ukai, M. Aoki, J. Takaya, N. Iwasawa, J. Am. Chem. Soc. 2006, 128, 8706–8707.
- 15H. Inomata, K. Ogata, S.-i. Fukuzawa, Z. Hou, Org. Lett. 2012, 14, 3986–3989.
- 16S. Li, W. Yuan, S. Ma, Angew. Chem. 2011, 123, 2626–2630; Angew. Chem. Int. Ed. 2011, 50, 2578–2582.
- 17K.-i. Tominaga, Y. Sasaki, K. Hagihara, T. Watanabe, M. Saito, Chem. Lett. 1994, 1391–1394.
- 18K.-i. Tominaga, Y. Sasaki, Catal. Commun. 2000, 1, 1–3.
- 19K.-i. Tominaga, Y. Sasaki, J. Mol. Catal. A 2004, 220, 159–165.
- 20S. Jääskeläinen, M. Haukka, Appl. Catal. A 2003, 247, 95–100.
- 21M.-L. Kontkanen, L. Oresmaa, M. A. Moreno, J. Jänis, E. Laurila, M. Haukka, Appl. Catal. A 2009, 365, 130–134.
- 22L. Wu, Q. Liu, I. Fleischer, D. Selent, R. Franke, R. Jackstell, M. Beller, Chem. Eur. J. 2014, DOI: .
- 23V. K. Srivastava, P. Eilbracht, Catal. Commun. 2009, 10, 1791–1795.
- 24L. Wu, Q. Liu, I. Fleischer, R. Jackstell, M. Beller, Nat. Commun. 2014, 5, 3091.
- 25T. G. Ostapowicz, M. Schmitz, M. Krystof, J. Klankermayer, W. Leitner, Angew. Chem. 2013, 125, 12341–12345;
10.1002/ange.201304529 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 12119–12123.
- 26C. M. Beck, S. E. Rathmill, Y. J. Park, J. Chen, R. H. Crabtree, L. M. Liable-Sands, A. L. Rheingold, Organometallics 1999, 18, 5311–5317.
- 27
- 27aJ. C. Leung, M. J. Krische, Chem. Sci. 2012, 3, 2202–2209;
- 27bM. C. Willis, Chem. Rev. 2010, 110, 725–748.
- 28T. Okano, T. Kobayashi, H. Konishi, J. Kiji, Tetrahedron Lett. 1982, 23, 4967–4968.
- 29H. S. Ahn, S. H. Han, S. J. Uhm, W. K. Seok, H. N. Lee, G. A. Korneeva, J. Mol. Catal. A 1999, 144, 295–306.
- 30M. Rosales, A. González, B. González, C. Moratinos, H. Pérez, J. Urdaneta, R. A. Sánchez-Delgado, J. Organomet. Chem. 2005, 690, 3095–3098.
- 31M. Rosales, F. Arrieta, P. Baricelli, Á. González, B. González, Y. Guerrero, C. Moratinos, I. Pacheco, H. Pérez, J. Urdaneta, Catal. Lett. 2008, 126, 367–370.
- 32G. Makado, T. Morimoto, Y. Sugimoto, K. Tsutsumi, N. Kagawa, K. Kakiuchi, Adv. Synth. Catal. 2010, 352, 299–304.
- 33E. Cini, E. Airiau, N. Girard, A. Mann, J. Salvadori, M. Taddei, Synlett 2011, 199–202.
- 34M. Uhlemann, S. Doerfelt, A. Börner, Tetrahedron Lett. 2013, 54, 2209–2211.
- 35A. Köpfer, B. Sam, B. Breit, M. J. Krische, Chem. Sci. 2013, 4, 1876.
- 36A. Behr, U. Kanne, W. Keim, J. Mol. Catal. 1986, 35, 19–28.
- 37J. Moran, A. Preetz, R. A. Mesch, M. J. Krische, Nat. Chem. 2011, 3, 287–290.
- 38J.-P. Simonato, T. Walter, P. Métivier, J. Mol. Catal. A 2001, 171, 91–94.
- 39M. G. Mura, L. D. Luca, G. Giacomelli, A. Porcheddu, Adv. Synth. Catal. 2012, 354, 3180–3186.
- 40W. Keim, J. Becker, J. Mol. Catal. 1989, 54, 95–101.
- 41P. Isnard, B. Denise, R. P. A. Sneeden, J. M. Cognion, P. Durual, J. Organomet. Chem. 1983, 256, 135–139.
- 42W. Ueda, T. Yokoyama, Y. Morikawa, Y. Moro-oka, T. Ikawa, J. Mol. Catal. 1988, 44, 197–200.
- 43E. M. Nahmed, G. Jenner, J. Mol. Catal. 1990, 59, L15–L19.
- 44G. Jenner, Tetrahedron Lett. 1991, 32, 505–508.
- 45G. Jenner, A. B. Taleb, J. Mol. Catal. 1994, 91, 31–43.
- 46G. Lavigne, N. Lugan, P. Kalck, J. M. Soulie, O. Lerouge, J. Y. Saillard, J. F. Halet, J. Am. Chem. Soc. 1992, 114, 10669–10670.
- 47
- 47aS. Fabre, P. Kalck, G. Lavigne, Angew. Chem. 1997, 109, 1167–1169;
10.1002/ange.19971091031 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1092–1095;
- 47bN. Lugan, G. Lavigne, J. M. Soulie, S. Fabre, P. Kalck, J. Y. Saillard, J. F. Halet, Organometallics 1995, 14, 1712–1731.
- 48C. Legrand, Y. Castanet, A. Mortreux, F. Petit, J. Chem. Soc. Chem. Commun. 1994, 1173–1174.
- 49S. Ko, Y. Na, S. Chang, J. Am. Chem. Soc. 2002, 124, 750–751.
- 50H. Konishi, T. Ueda, T. Muto, K. Manabe, Org. Lett. 2012, 14, 4722–4725.
- 51I. J. B. Lin, H. Alper, J. Chem. Soc. Chem. Commun. 1989, 248–249.
- 52J. Grévin, P. Kalck, J. Organomet. Chem. 1994, 476, c 23–c24.
- 53I. Fleischer, R. Jennerjahn, D. Cozzula, R. Jackstell, R. Franke , M. Beller, ChemSusChem 2013, 6, 417–420.
- 54N. Armanino, M. Lafrance, E. M. Carreira, Org. Lett. 2013, 16, 572–575.
- 55Y. Tsuji, S. Yoshii, T. Ohsumi, T. Kondo, Y. Watanabe, J. Organomet. Chem. 1987, 331, 379–385.
- 56T. Kondo, T. Okada, T.-a. Mitsudo, Organometallics 1999, 18, 4123–4127.
- 57S. Ko, H. Han, S. Chang, Org. Lett. 2003, 5, 2687–2690.
- 58J. C. Serrano-Ruiz, R. Luque, A. Sepulveda-Escribano, Chem. Soc. Rev. 2011, 40, 5266–5281.
- 59
- 59aK. Takahashi, M. Yamashita, Y. Tanaka, K. Nozaki, Angew. Chem. 2012, 124, 4459–4463; Angew. Chem. Int. Ed. 2012, 51, 4383–4387;
- 59bI. Fleischer, K. M. Dyballa, R. Jennerjahn, R. Jackstell, R. Franke, A. Spannenberg, M. Beller, Angew. Chem. 2013, 125, 3021–3025;
10.1002/ange.201207133 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 2949–2953;
- 59cL. Wu, I. Fleischer, R. Jackstell, I. Profir, R. Franke, M. Beller, J. Am. Chem. Soc. 2013, 135, 14306–14312.