Geometric Requirements for Hydrocarbon Catalytic Sites on Platinum Surfaces†
The work by Bruce Koel at Princeton University and Simon Podkolzin at Stevens Institute of Technology was supported by the National Science Foundation under, respectively, grants CHE-1129417 and CBET-1264453. The Materials Studio software was used under a collaborative research license from Accelrys Software, Inc.
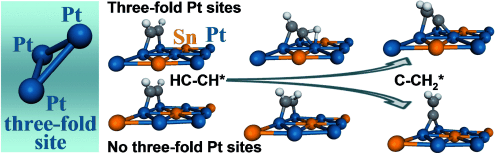
Graphical Abstract
A preferential catalytic mechanism has been identified for the transformation of acetylene to vinylidene on Pt-Sn surfaces. Unlike a direct H shift along the CC bond in organometallic compounds, this mechanism requires three adjacent Pt atoms. The same requirement is identified for CH bond cleavage. Without three-fold Pt sites, the reaction mechanism changes, and reactions of H transfer and CH bond cleavage are suppressed.
Abstract
Vibrational spectroscopic measurements and density functional calculations were used to identify a preferential catalytic mechanism for the transformation of acetylene, HCCH, to vinylidene, CCH2, on surfaces of Pt-Sn ordered alloys. In this mechanism, two adjacent Pt atoms adsorb an acetylene molecule and a third neighboring Pt atom is required for stabilizing the reacting H atom during the transformation. Therefore, unlike a direct H shift along the CC bond in organometallic compounds with a single transition-metal atom, this mechanism has a geometric site requirement of three adjacent Pt atoms in the form of a three-fold site. The same geometric site requirement is identified for preferential CH bond cleavage of acetylene with the formation of adsorbed CCH and H species. In the absence of three-fold Pt sites, the reaction mechanism changes, and reactions of H transfer and CH bond cleavage are suppressed.