Heteroatom-Free Arene-Cobalt and Arene-Iron Catalysts for Hydrogenations
M. Sc. Dominik Gärtner
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Search for more papers by this authorDr. Alice Welther
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Search for more papers by this authorDr. Babak Rezaei Rad
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Robert Wolf
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)Search for more papers by this authorCorresponding Author
Prof. Dr. Axel Jacobi von Wangelin
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)Search for more papers by this authorM. Sc. Dominik Gärtner
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Search for more papers by this authorDr. Alice Welther
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Search for more papers by this authorDr. Babak Rezaei Rad
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Robert Wolf
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)Search for more papers by this authorCorresponding Author
Prof. Dr. Axel Jacobi von Wangelin
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)
Fakultät für Chemie, Universität Regensburg, Universitätsstrasse 31, 93040 Regensburg (Germany)Search for more papers by this authorGraphical Abstract
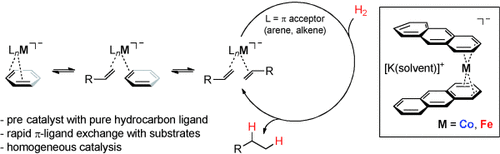
Especially high selectivities were observed in the hydrogenation of various alkenes, ketones, and imines with bis(anthracene)cobaltate(−I) [K(dme)2{Co(C14H10)2}] under mild conditions (1–5 mol % cat., 1–10 bar H2, 20–60 °C). Mechanistic studies indicate the operation in alkene hydrogenations of a homogeneous catalyst formed by initial ligand exchange and stabilized by the coordination of π-acidic alkenes or arenes.
Abstract
75 years after the discovery of hydroformylation, cobalt catalysts are now undergoing a renaissance in hydrogenation reactions. We have evaluated arene metalates in which the low-valent metal species is—conceptually different from heteroatom-based ligands—stabilized by π coordination to hydrocarbons. Potassium bis(anthracene)cobaltate 1 and -ferrate 2 can be viewed as synthetic precursors of quasi-“naked” anionic metal species; their aggregation is effectively impeded by (labile) coordination to the various π acceptors present in the hydrogenation reactions of unsaturated molecules (alkenes, arenes, carbonyl compounds). Kinetic studies, NMR spectroscopy, and poisoning studies of alkene hydrogenations support the formation of a homogeneous catalyst derived from 1 which is stabilized by the coordination of alkenes. This catalyst concept complements the use of complexes with heteroatom donor ligands for reductive processes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201308967_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Catalytic Hydrogenation (Ed.: ), Elsevier, Amsterdam, 1986;
- 1b The Handbook of Homogeneous Hydrogenation (Eds.: ), Wiley-VCH, Weinheim, 2007.
- 2
- 2aP. N. Rylander, Catalytic Hydrogenation over Platinum Metals, Academic Press, New York, 1967;
- 2bA. Molnár, A. Sárkány, M. Varga, J. Mol. Catal. A 2001, 173, 185;
- 2cH. Lindlar, Helv. Chim. Acta 1952, 35, 446.
- 3
- 3a Catalysis without Precious Metals (Ed.: ), Wiley-VCH, Weinheim, 2010;
- 3bM. S. Holzwarth, B. Plietker, ChemCatChem 2013, 5, 1650;
- 3cK. Junge, K. Schröder, M. Beller, Chem. Commun. 2011, 47, 4849;
- 3dR. H. Morris, Chem. Soc. Rev. 2009, 38, 2282.
- 4
- 4aG. Ertl, Catal. Rev. Sci. Eng. 1980, 21, 201;
- 4bG. P. van der Laan, A. A. C. M. Beenackers, Catal. Rev. Sci. Eng. 1999, 41, 255.
- 5
- 5aL. H. Slaugh, R. D. Mullineaux, US Patent 3239569, 1966;
- 5bJ. L. van Winkle, R. C. Morris, R. F. Mason, US Patent 3420898, 1969;
- 5cG. F. Pregaglia, A. Andreetta, G. F. Ferrari, R. Ugo, J. Organomet. Chem. 1971, 30, 387;
- 5dG. F. Ferrari, A. Andreetta, G. F. Pregaglia, R. Ugo, J. Organomet. Chem. 1972, 43, 209;
- 5eH. M. Feder, J. Halpern, J. Am. Chem. Soc. 1975, 97, 7186.
- 6Selected examples:
- 6aM. R. Thompson, V. W. Day, K. D. Tau, E. L. Muetterties, Inorg. Chem. 1981, 20, 1237;
- 6bQ. Knijnenburg, A. D. Horton, H. v. d. Heijden, T. M. Kooistra, D. G. H. Hetterscheid, J. M. M. Smits, B. de Bruin, P. H. M. Budzelaar, A. W. Gal, J. Mol. Catal. A 2005, 232, 151;
- 6cQ. Knijnenburg, D. Hetterscheid, T. M. Kooistra, P. H. M. Budzelaar, Eur. J. Inorg. Chem. 2004, 1204;
- 6dE. J. Daida, J. C. Peters, Inorg. Chem. 2004, 43, 7474–7485;
- 6eS. C. Bart, E. J. Hawrelak, E. Lobkovsky, P. J. Chirik, Organometallics 2005, 24, 5518;
- 6fM. Amézquita-Valencia, A. Cabrera, J. Mol. Catal. A 2013, 366, 17;
- 6gW. Zuo, A. J. Lough, Y. F. Li, R. H. Morris, Science 2013, 342, 1080;
- 6hJ. F. Sonnenberg, N. Coombs, P. A. Dube, R. H. Morris, J. Am. Chem. Soc. 2012, 134, 5893.
- 7
- 7aG. Zhang, B. L. Scott, S. K. Hanson, Angew. Chem. 2012, 124, 12268; Angew. Chem. Int. Ed. 2012, 51, 12102;
- 7bG. Zhang, K. V. Vasudevan, B. L. Scott, S. K. Hanson, J. Am. Chem. Soc. 2013, 135, 8668.
- 8S. Monfette, Z. R. Turner, S. P. Semproni, P. J. Chirik, J. Am. Chem. Soc. 2012, 134, 4561.
- 9
- 9aS. C. Bart, E. Lobkovsky, P. J. Chirik, J. Am. Chem. Soc. 2004, 126, 13794;
- 9bR. J. Trovitch, E. Lobkovsky, E. Bill, P. J. Chirik, Organometallics 2008, 27, 1470;
- 9cS. K. Russell, C. Milsmann, E. Lobkovsky, T. Weyhermüller, P. J. Chirik, Inorg. Chem. 2011, 50, 3159;
- 9dR. Yu, J. M. Darmon, C. Milsmann, G. W. Margulieux, S. C. E. Stieber, S. DeBeer, P. J. Chirik, J. Am. Chem. Soc. 2013, 135, 13168;
- 9easymmetric hydrogenation: M. R. Friedfeld, M. Shevlin, J. Hoyt, S. W. Krska, M. Tudge, P. J. Chirik, Science 2013, 342, 1076.
- 10
- 10aW. W. Brennessel, J. V. G. Young, J. E. Ellis, Angew. Chem. 2002, 114, 1259;
10.1002/1521-3757(20020402)114:7<1259::AID-ANGE1259>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1211;10.1002/1521-3773(20020402)41:7<1211::AID-ANIE1211>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 10bJ. E. Ellis, Inorg. Chem. 2006, 45, 3167;
- 10cW. W. Brennessel, R. E. Jilek, J. E. Ellis, Angew. Chem. 2007, 119, 6244;
10.1002/ange.200701353 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6132;
- 10dW. W. Brennessel, J. E. Ellis, Inorg. Chem. 2012, 51, 9076.
- 11
- 11aR. Wolf, A. W. Ehlers, J. C. Slootweg, M. Lutz, D. Gudat, M. Hunger, A. L. Spek, K. Lammertsma, Angew. Chem. 2008, 120, 4660; Angew. Chem. Int. Ed. 2008, 47, 4584;
- 11bR. Wolf, J. C. Slootweg, A. W. Ehlers, F. Hartl, B. de Bruin, M. Lutz, A. L. Spek, K. Lammertsma, Angew. Chem. 2009, 121, 3150; Angew. Chem. Int. Ed. 2009, 48, 3104;
- 11cR. Wolf, N. Ghavtadze, K. Weber, E.-M. Schnöckelborg, B. de Bruin, A. W. Ehlers, K. Lammertsma, Dalton Trans. 2010, 39, 1453.
- 12J. B. Johnson, T. Rovis, Angew. Chem. 2008, 120, 852;
10.1002/ange.200700278 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 840.
- 13For applications of the same concept in cross-coupling reactions, see
- 13aS. Gülak, O. Stepanek, J. Malberg, B. R. Rad, M. Kotora, R. Wolf, A. Jacobi von Wangelin, Chem. Sci. 2013, 4, 776;
- 13bK. Weber, E.-M. Schnöckelborg, R. Wolf, ChemCatChem 2011, 3, 1572.
- 14Examples of arene coordination in catalyic olefin hydrogenation:
- 14aD. Heller, H. J. Drexler, A. Spannenberg, B. Heller, J. You, W. Baumann, Angew. Chem. 2002, 114, 814;
10.1002/1521-3757(20020301)114:5<814::AID-ANGE814>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 2002, 41, 777;10.1002/1521-3773(20020301)41:5<777::AID-ANIE777>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 14bJ. Halpern, D. P. Riley, A. S. C. Chan, J. J. Pluth, J. Am. Chem. Soc. 1977, 99, 8055.
- 15Polymerization of styrenes: A. Hirao, S. Loykulnant, T. Ishizone, Prog. Polym. Sci. 2002, 27, 1399.
- 16Recent procedures from our research group:
- 16aligand-free Fe-catalyzed hydrogenation of styrenes: A. Welther, M. Bauer, M. Mayer, A. Jacobi von Wangelin, ChemCatChem 2012, 4, 1088;
- 16bFe-catalyzed semi-hydrogenation of alkynes: T. N. Gieshoff, A. Welther, M. T. Kessler, M. H. G. Prechtl, A. Jacobi von Wangelin, Chem. Commun. 2014, 50, 2261–2264.
- 17
- 17aG. Cahiez, A. Moyeux, Chem. Rev. 2010, 110, 1435;
- 17bW. M. Czaplik, M. Mayer, J. Cvengros, A. Jacobi von Wangelin, ChemSusChem 2009, 2, 396.
- 18
- 18aW. A. Herrmann, M. Prinz, Applied Homogeneous Catalysis with Organometallic Compounds, Vol. 3, 2nd ed. (Eds.: ), Wiley-VCH, Weinheim, 2002, p. 1119;
10.1002/9783527618231 Google Scholar
- 18bT. Kobayashi, H. Yorimitsu, K. Oshima, Chem. Asian J. 2009, 4, 1078;
- 18cG. Fachinetti, A. Stefani, Angew. Chem. 1982, 94, 937;
10.1002/ange.19820941231 Google ScholarAngew. Chem. Int. Ed. Engl. 1982, 21, 925;
- 18dM. Bianchi, F. Paicenti, P. Frediani, U. Matteoli, J. Organomet. Chem. 1977, 137, 361;
- 18eB. Fell, W. Rupilius, F. Asinger, Tetrahedron Lett. 1968, 9, 3261.
10.1016/S0040-4039(00)89542-8 Google Scholar
- 19A. Panda, M. Stender, R. J. Wright, M. M. Olmstead, P. Klavins, P. P. Power, Inorg. Chem. 2002, 41, 3909.
- 20J. Vela, S. Stoian, C. J. Flaschenriem, E. Münck, P. L. Holland, J. Am. Chem. Soc. 2004, 126, 4522.
- 21
- 21aJ. A. Widegren, R. G. Finke, J. Mol. Catal. A 2003, 198, 317;
- 21bD. Astruc, F. Lu, J. R. Aranzaes, Angew. Chem. 2005, 117, 8062; Angew. Chem. Int. Ed. 2005, 44, 7852;
- 21cR. H. Crabtree, Chem. Rev. 2012, 112, 1536.
- 22
- 22aD. R. Anton, R. H. Crabtree, Organometallics 1983, 2, 855;
- 22bG. Franck, M. Brill, G. Helmchen, J. Org. Chem. 2012, 89, 55.
- 23K. P. Tellmann, M. J. Humphries, H. S. Rzepa, V. C. Gibson, Organometallics 2004, 23, 5503.
- 24
- 24aIn the presence of 1 mol % 1 and 20 mol % 1,3-diphenyl-2-propanol, styrenes were hydrogenated much slower than with 1 (see the Supporting Information);
- 24bthe oxidation of the catalyst by alcohols might also lead to partial formation of Co0 nanoparticles. So far, poisoning experiments with Hg and dct were not conclusive.
- 25Selected examples of cobalt(I)-catalyzed olefin hydrogenations:
- 25aT.-P. Lin, J. C. Peters, J. Am. Chem. Soc. 2013, 135, 15310;
- 25bN. Weding, A. Spannenberg, R. Jackstell, M. Hapke, Organometallics 2012, 31, 5660;
- 25cK. Kawakami, T. Mizoroki, A. Ozaki, J. Mol. Catal. 1979, 5, 175;
- 25dH. Kanai, N. Yamamoto, K. Kishi, K. Mizuno, K. Tarama, J. Catal. 1982, 73, 228.
- 26Dehydrogenations of ethyl dihydrocinnamate and 2-ethylnaphthalene were not observed with 5 mol % 1 at 60 °C, respectively. See Ref. [7b].