Asymmetric Diboration of Terminal Alkenes with a Rhodium Catalyst and Subsequent Oxidation: Enantioselective Synthesis of Optically Active 1,2-Diols†
Kenji Toribatake
Department of Applied Chemistry, Graduated School of Engineering, Nagoya University, Chikusa, Nagoya, 464-8603 (Japan) http://www.apchem.nagoya-u.ac.jp/06-II-1/nisilab/index.html
Search for more papers by this authorCorresponding Author
Prof. Dr. Hisao Nishiyama
Department of Applied Chemistry, Graduated School of Engineering, Nagoya University, Chikusa, Nagoya, 464-8603 (Japan) http://www.apchem.nagoya-u.ac.jp/06-II-1/nisilab/index.html
Department of Applied Chemistry, Graduated School of Engineering, Nagoya University, Chikusa, Nagoya, 464-8603 (Japan) http://www.apchem.nagoya-u.ac.jp/06-II-1/nisilab/index.htmlSearch for more papers by this authorKenji Toribatake
Department of Applied Chemistry, Graduated School of Engineering, Nagoya University, Chikusa, Nagoya, 464-8603 (Japan) http://www.apchem.nagoya-u.ac.jp/06-II-1/nisilab/index.html
Search for more papers by this authorCorresponding Author
Prof. Dr. Hisao Nishiyama
Department of Applied Chemistry, Graduated School of Engineering, Nagoya University, Chikusa, Nagoya, 464-8603 (Japan) http://www.apchem.nagoya-u.ac.jp/06-II-1/nisilab/index.html
Department of Applied Chemistry, Graduated School of Engineering, Nagoya University, Chikusa, Nagoya, 464-8603 (Japan) http://www.apchem.nagoya-u.ac.jp/06-II-1/nisilab/index.htmlSearch for more papers by this authorThis research was partly supported by the Japan Society for the Promotion of Science (No. 2562007).
Graphical Abstract
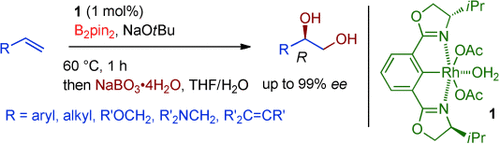
Pin it down: A highly enantioselective diboration of terminal alkenes with chiral 1 and bis(pinacolato)diboron (B2pin2) was realized. Subsequent oxidation of the diboron adducts with sodium peroxoborate readily gave the corresponding optically active 1,2-diols in high yields and high enantioselectivities.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305181_sm_miscellaneous_information.pdf14.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected papers:
- 1aA. B. Smith III, S. S.-Y. Chen, F. C. Nelson, J. M. Reichert, B. A. Salvatore, J. Am. Chem. Soc. 1995, 117, 12013;
- 1bP. J. Pye, K. Rossen, S. A. Weissman, A. Maliakal, R. A. Reamer, R. Ball, N. N. Tsou, R. P. Volante, P. J. Reider, Chem. Eur. J. 2002, 8, 1372;
10.1002/1521-3765(20020315)8:6<1372::AID-CHEM1372>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 1cS. H. Kang, J. W. Jeong, Y. S. Hwang, S. B. Lee, Angew. Chem. 2002, 114, 1450;
Angew. Chem. Int. Ed. 2002, 41, 1392;
10.1002/1521-3773(20020415)41:8<1392::AID-ANIE1392>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 1dP. Gupta, S. V. Naidu, P. Kumar, Tetrahedron Lett. 2004, 45, 849;
- 1eB. J. Edagwa, C. M. Taylor, J. Org. Chem. 2009, 74, 4132;
- 1fB. B. Lohray, A. S. Reddy, V. Bhushan, Tetrahedron: Asymmetry 1996, 7, 2411;
- 1gL. M. Schultze, H. H. Chapman, N. J. P. Dubree, R. J. Jones, K. M. Kent, T. T. Lee, M. S. Louie, M. J. Postich, E. J. Prise, J. C. Rohloff, R. H. Yu, Tetrahedron Lett. 1998, 39, 1853.
- 2
- 2aH. C. Kolb, M. S. Van Nieuwenhze, K. B. Sharpless, Chem. Rev. 1994, 94, 2483;
- 2bM. C. Noe, M. A. Letavic, S. L. Show, Org. React. 2005, 66, 109–625;
- 2cA. B. Zaitsev, H. Adolfsson, Synthesis 2006, 1725;
- 2dS. Kobayashi, M. Sugiura, Adv. Synth. Catal. 2006, 348, 1496.
- 3For examples, asymmetric reduction of α-hydroxyketones:
- 3aT. Ohkuma, N. Utsumi, M. Watanabe, K. Tsutsumi, N. Arai, K. Murata, Org. Lett. 2007, 9, 2565;
- 3bR. Kadyrov, R. M. Koenigs, C. Brinkmann, D. Voigtlaender, M. Rueping, Angew. Chem. 2009, 121, 7693;
10.1002/ange.200902835 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7556;
- 3cT. Saito, T. Yokozawa, T. Ishizaki, T. Moroi, N. Sayo, T. Miura, H. Kumobayashi, Adv. Synth. Catal. 2001, 343, 264;
- 3dM. Kitamura, T. Ohkuma, S. Inoue, N. Sayo, H. Kumobayashi, S. Akutagawa, T. Ohta, H. Takaya, R. Noyori, J. Am. Chem. Soc. 1988, 110, 629;
- 3eS. Jeulin, N. Champion, S. Duprat de Paule, P. Dellis, V. Ratovelmanana-Vidal, J.-P. Genêt, Synthesis 2005, 3666. For transfer hydrogenation of α-hydroxyketones, see
- 3fD. J. Cross, J. A. Kenny, I. Houson, L. Campbell, T. Walsgrove, M. Wills, Tetrahedron: Asymmetry 2001, 12, 1801;
- 3gD. S. Matharu, D. J. Morris, A. M. Kawamoto, G. J. Clarkson, M. Wills, Org. Lett. 2005, 7, 5489;
- 3hT. Hamada, T. Torii, K. Izawa, T. Ikariya, Tetrahedron 2004, 60, 7411.
- 4
- 4aM. Tokunaga, J. F. Larrow, F. Kakiuchi, E. N. Jacobsen, Science 1997, 277, 936;
- 4bS. E. Schaus, B. D. Brandes, J. F. Larrow, M. Tokunaga, K. B. Hansen, A. E. Gould, M. E. Furrow, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 1307.
- 5For a review, see:
- 5aN. Bala, S. S. Chimni, Tetrahedron: Asymmetry 2010, 21, 2879. Selected achievements of microbial reactions:
- 5bY. Nie, Y. Xu, X. Q. Mu, Org. Process Res. Dev. 2004, 8, 246;
- 5cZ. Liu, J. Michel, Z. Wang, B. Witholt, Z. Li, Tetrahedron: Asymmetry 2006, 17, 47;
- 5dS. Hwang, C. Y. Choi, E. Y. Lee, Biotechnol. Lett. 2008, 30, 1219;
- 5eP. Mahajabeen, A. Chadha, Tetrahedron: Asymmetry 2011, 22, 2156.
- 6C. J. R. Bataille, T. J. Donohoe, Chem. Soc. Rev. 2011, 40, 114.
- 7For selected achievements:
- 7aK. Suzuki, P. D. Oldenburg, L. Que, Jr., Angew. Chem. 2008, 120, 1913; Angew. Chem. Int. Ed. 2008, 47, 1887;
- 7bT. W.-S. Chow, E. L.-M. Wong, Z. Guo, Y. Liu, J.-S. Huang, C.-M. Che, J. Am. Chem. Soc. 2010, 132, 13229.
- 8For reviews of catalytic diboration including asymmetric reactions, see:
- 8aI. Beletskaya, C. Moberg, Chem. Rev. 2006, 106, 2320;
- 8bH. E. Burks, J. P. Morken, Chem. Commun. 2007, 4717.
- 9For achievements of catalytic diboration:
- 9aR. T. Baker, P. Nguyen, T. B. Marder, S. A. Westcott, Angew. Chem. 1995, 107, 1451; Angew. Chem. Int. Ed. Engl. 1995, 34, 1336;
- 9bC. Dai, E. G. Robins, A. J. Scott, W. Clegg, D. S. Yufit, J. A. K. Howard, T. B. Marder, Chem. Commun. 1998, 1983;
- 9cP. Nugyen, R. B. Coapes, A. D. Woodward, N. J. Taylor, J. M. Burke, J. A. K. Howard, T. B. Marder, J. Organomet. Chem. 2002, 652, 77;
- 9dT. Ishiyama, M. Yamamoto, N. Miyaura, Chem. Commun. 1997, 689;
- 9eC. N. Iverson, M. R. Smith III, Organometallics 1997, 16, 2757;
- 9fJ. Ramírez, R. Corberán, M. Sanaú, E. Peris, E. Fernandez, Chem. Commun. 2005, 3056;
- 9gR. Corberán, J. Ramírez, M. Poyatos, E. Peris, E. Fernández, Tetrahedron: Asymmetry 2006, 17, 1759;
- 9hV. Lillo, M. R. Fructos, J. Ramírez, A. A. C. Braga, F. Maseras, M. M. Díaz-Requejo, P. J. Pérez, E. Fernández, Chem. Eur. J. 2007, 13, 2614. For a theoretical approach, see:
- 9iL. Dang, H. Zhao, Z. Lin, T. B. Marder, Organometallics 2007, 26, 2824;
- 9jL. Dang, H. Zhao, Z. Lin, T. B. Marder, Organometallics 2008, 27, 1178. For asymmetric induction, see:
- 9kT. B. Marder, N. C. Norman, C. R. Rice, Tetrahedron Lett. 1998, 39, 155.
- 10
- 10aJ. B. Morgan, S. P. Miller, J. P. Morken, J. Am. Chem. Soc. 2003, 125, 8702;
- 10bS. Trudeau, J. B. Morgan, M. Shrestha, J. P. Morken, J. Org. Chem. 2005, 70, 9538;
- 10cS. P. Miller, J. B. Morgan, F. J. Nepveux, J. P. Morken, Org. Lett. 2004, 6, 131.
- 11
- 11aL. T. Kliman, S. N. Mlynarski, J. P. Morken, J. Am. Chem. Soc. 2009, 131, 13210; for dienes as substrates:
- 11bN. F. Pelz, A. R. Woodward, H. E. Burks, J. D. Sieber, J. P. Morken, J. Am. Chem. Soc. 2004, 126, 16328;
- 11cH. E. Burks, L. T. Kliman, J. P. Morken, J. Am. Chem. Soc. 2009, 131, 9134;
- 11dK. Hong, J. P. Morken, J. Org. Chem. 2011, 76, 9102;
- 11eL. T. Kliman, S. N. Mlynarski, G. E. Ferris, J. P. Morken, Angew. Chem. 2012, 124, 536;
10.1002/ange.201105716 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 521;
- 11fG. E. Ferris, K. Hong, I. A. Roundtree, J. P. Morken, J. Am. Chem. Soc. 2013, 135, 2501.
- 12For reviews of catalytic conjugate boration including asymmetric reactions, see:
- 12aV. Lillo, A. Bonet, E. Fernández, Dalton Trans. 2009, 2899;
- 12bL. Dang, Z. Lin, T. B. Marder, Chem. Commun. 2009, 3987;
- 12cJ. A. Schiffner, K. Müther, M. Oestreich, Angew. Chem. 2010, 122, 1214;
10.1002/ange.200906521 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1194;
- 12dE. Hartmann, D. J. Vyas, M. Oestreich, Chem. Commun. 2011, 47, 7917;
- 12eA. D. J. Calow, A. Whiting, Org. Biomol. Chem. 2012, 10, 5485. For other reviews of catalytic boration:
- 12fT. Ohmura, M. Suginome, Bull. Chem. Soc. Jpn. 2009, 82, 29;
- 12gJ. Takaya, N. Iwasawa, ACS Catal. 2012, 2, 1993.
- 13
- 13aK. Lee, A. R. Zhugralin, A. H. Hoveyda, J. Am. Chem. Soc. 2009, 131, 7253;
- 13bA. Bonet, H. Gulyás, E. Fernández, Angew. Chem. 2010, 122, 5256;
10.1002/ange.201001198 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5130;
- 13cC. Pubill-Ulldemolins, A. Bonet, C. Bo, H. Gulyás, E. Fernández, Chem. Eur. J. 2012, 18, 1121;
- 13dH. Wu, S. Radomkit, J. M. O’Brien, A. H. Hoveyda, J. Am. Chem. Soc. 2012, 134, 8277.
- 14
- 14aA. Bonet, C. Pubill-Ulldemolins, C. Bo, H. Gulyás, E. Fernández, Angew. Chem. 2011, 123, 7296;
10.1002/ange.201101941 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7158;
- 14bA. Bonet, C. Sole, H. Gulyás, E. Fernández, Org. Biomol. Chem. 2012, 10, 6621.
- 15
- 15aT. Shiomi, T. Adachi, K. Toribatake, L. Zhou, H. Nishiyama, Chem. Commun. 2009, 5987;
- 15bK. Toribatake, L. Zhou, A. Tsuruta, H. Nishiyama, Tetrahedron 2013, 69, 3551. For preparation of [Rh(Phebox)] catalysts, see:
- 15cY. Kanazawa, Y. Tsuchiya, K. Kobayashi, T. Shiomi, J. Itoh, M. Kikuchi, Y. Yamamoto, H. Nishiyama, Chem. Eur. J. 2006, 12, 63;
- 15dJ. Ito, T. Shiomi, H. Nishiyama, Adv. Synth. Catal. 2006, 348, 1235. For reviews on applications of [Rh(Phebox)] catalysts, see:
- 15eH. Nishiyama, J. Ito, Chem. Commun. 2010, 46, 203;
- 15fJ. Ito, H. Nishiyama, Synlett 2012, 509.
- 16C. Pubill-Ulldemolins, M. Poyatos, C. Bo, E. Fernández, Dalton Trans. 2013, 42, 746. Rh/NHC-catalyzed diboration and theoretical explanation.
- 17T. Shimada, K. Mukaide, A. Shinohara, J. W. Han, T. Hayashi, J. Am. Chem. Soc. 2002, 124, 1584.