Asymmetric Hydrogenation of Ketones with H2 and Ruthenium Catalysts Containing Chiral Tetradentate S2N2 Ligands†
Ruth Patchett
Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorIris Magpantay
Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorDr. Lionel Saudan
Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorDr. Christoph Schotes
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Antonio Mezzetti
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich (Switzerland)
Antonio Mezzetti, Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich (Switzerland)
Francesco Santoro, Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorCorresponding Author
Dr. Francesco Santoro
Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Antonio Mezzetti, Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich (Switzerland)
Francesco Santoro, Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorRuth Patchett
Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorIris Magpantay
Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorDr. Lionel Saudan
Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorDr. Christoph Schotes
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Antonio Mezzetti
Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich (Switzerland)
Antonio Mezzetti, Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich (Switzerland)
Francesco Santoro, Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorCorresponding Author
Dr. Francesco Santoro
Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Antonio Mezzetti, Department of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich (Switzerland)
Francesco Santoro, Corporate R&D Division, Firmenich SA route des Jeunes 1, P.O. Box 239, 1211 Genève 8 (Switzerland)
Search for more papers by this authorThe Authors thank Mr. Rino Schwenk for his assistance with the X-ray structure of complex 2 a and Dr. Alec Birkbeck for the generous donation of substrate 3 l.
Graphical Abstract
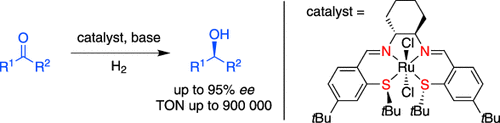
Getting more for less: In the presence of H2 and a base, air- and moisture-tolerant RuII complexes catalyze the hydrogenation of ketones and aldehydes with excellent activity and chemoselectivity, and with enantioselectivity of up to 95 % under mild conditions. The ratio of substrate to catalyst can be lowered to 106:1. The reactions tolerate scale-up and can be carried out with almost no solvent. A base-free method is available for base-sensitive substrates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304844_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For seminal papers, see:
- 1aR. Noyori, T. Ohkuma, M. Kitamura, N. Sayo, H. Kumobayashi, S. Akutagawa, J. Am. Chem. Soc. 1987, 109, 5856–5858;
- 1bM. Kitamura, T. Ohkuma, S. Inoue, N. Sayo, H. Kumobayashi, S. Akutagawa, T. Ohta, H. Takaya, R. Noyori, J. Am. Chem. Soc. 1988, 110, 629–631;
- 1cT. Ohkuma, H. Ooka, S. Hashiguchi, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 1995, 117, 2675–2676;
- 1dH. Doucet, T. Ohkuma, K. Murata, T. Yokozawa, M. Kozawa, E. Katayama, A. F. England, T. Ikariya, R. Noyori, Angew. Chem. 1998, 110, 1792–1796;
10.1002/(SICI)1521-3757(19980619)110:12<1792::AID-ANGE1792>3.0.CO;2-9 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1703–1707; for a review article, see:10.1002/(SICI)1521-3773(19980703)37:12<1703::AID-ANIE1703>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 1eR. Noyori, T. Ohkuma, Angew. Chem. 2001, 113, 40–75;
10.1002/1521-3757(20010105)113:1<40::AID-ANGE40>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2001, 40, 40–73;
- 1fR. Noyori, Angew. Chem. 2002, 114, 2108–2123;
10.1002/1521-3757(20020617)114:12<2108::AID-ANGE2108>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2008–2022;10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 1gH.-U. Blaser, C. Malan, B. Pugin, F. Spindler, H. Steiner, M. Studer, Adv. Synth. Catal. 2003, 345, 103–151;
- 1h The Handbook of Homogeneous Hydrogenation (Eds.: ), Wiley-VCH, Weinheim, 2006;
- 1iC. Hedberg in Modoths in Reduction Methods (Eds.: ), Wiley-VCH, Weinheim, 2008, pp. 107–134;
10.1002/9783527622115.ch5 Google Scholar
- 1jP. Dupau, Top. Organomet. Chem. 2012, 42, 47–64.
- 2W. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029–3070.
- 3M. Ito, M. Hirakawa, K. Murata, T. Ikariya, Organometallics 2001, 20, 379–381.
- 4
- 4aT. Ohkuma, N. Utsumi, K. Tsutsumi, K. Murata, C. A. Sandoval, R. Noyori, J. Am. Chem. Soc. 2006, 128, 8724–8725;
- 4bC. A. Sandoval, T. Ohkuma, N. Utsumi, K. Tsutsumi, K. Murata, R. Noyori, Chem. Asian J. 2006, 1, 102–110;
- 4cT. Ohkuma, K. Tsutsumi, N. Utsumi, N. Arai, R. Noyori, K. Murata, Org. Lett. 2007, 9, 255–257;
- 4dC. A. Sandoval, F. Bie, A. Matsuoka, Y. Yamaguchi, H. Naka, Y. Li, K. Kato, N. Utsumi, K. Tsutsumi, T. Ohkuma, K. Murata, R. Noyori, Chem. Asian J. 2010, 5, 806–816.
- 5
- 5aJ. I. Ito, S. Ujiie, H. Nishiyama, Chem. Commun. 2008, 1923–1925;
- 5bJ. I. Ito, S. Ujiie, H. Nishiyama, Organometallics 2009, 28, 630–638;
- 5cJ. I. Ito, T. Teshima, H. Nishiyama, Chem. Commun. 2012, 48, 1105–1107.
- 6For general reviews on transfer hydrogenation, see:
- 6aG. Zassinovich, G. Mestroni, S. Gladiali, Chem. Rev. 1992, 92, 1051–1069;
- 6bR. Noyori, S. Hashiguchi, Acc. Chem. Res. 1997, 30, 97–102;
- 6cT. Ikariya, A. J. Blacker, Acc. Chem. Res. 2007, 40, 1300–1308;
- 6dC. Wang, X. F. Wu, J. L. Xiao, Chem. Asian J. 2008, 3, 1750–1770; for an example using chiral N,S-bidentate ligands, see:
- 6eM. Ito, Y. Shibata, A. Watanabe, T. Ikariya, Synlett 2009, 1621–1626.
- 7
- 7aV. Rautenstrauch, R. Churlaud, R. H. Morris, K. Abdur-Rashid, WO 2002/040155, 2002;
- 7bV. Rautenstrauch, X. Hoang-Cong, R. Churlaud, K. Abdur-Rashid, R. H. Morris, Chem. Eur. J. 2003, 9, 4954–4967.
- 8J. X. Gao, T. Ikariya, R. Noyori, Organometallics 1996, 15, 1087–1089.
- 9RuII/PNNP complexes contain the same P2N2 donor set as the RuII/diamine/diphosphine catalysts, which are the most efficient catalysts for asymmetric ketone hydrogenation; see Ref. [1].
- 10F. Santoro, L. Saudan, C. Saudan, WO 2012/084810, 2012.
- 11For the synthesis of other Ru/SNNS complexes, see:
- 11aK. Nakajima, Y. Ando, H. Mano, M. Kojima, Inorg. Chim. Acta 1998, 274, 184–191;
- 11bT. Yamamura, M. Tadokoro, R. Kuroda, Chem. Lett. 1989, 18, 1245–1246.
- 12Chiral thiophene-based SNNS ligands were reported in the Ir-catalyzed asymmetric TRHY of ketones; however, the thiophene donors were not involved in coordination to the metal center; see:
- 12aX. Q. Zhang, Y. Y. Li, H. Zhang, J. X. Gao, Tetrahedron: Asymmetry 2007, 18, 2049–2054;
- 12bX. Q. Zhang, Y. Y. Li, Z. R. Dong, W. Y. Shen, Z. B. Cheng, J. X. Gao, J. Mol. Catal. A 2009, 307, 149–153; for an achiral Ru/SNS catalyst for the hydrogenation of esters, ketones, aldehydes, imines, and olefins, see:
- 12cD. Spasyuk, S. Smith, D. G. Gusev, Angew. Chem. 2013, 125, 2598–2602;
10.1002/ange.201209218 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 2538–2542.
- 13See, for example: C. Sui-Seng, F. Freutel, A. J. Lough, R. H. Morris, Angew. Chem. 2008, 120, 954–957;
10.1002/ange.200705115 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 940–943.
- 14T. Katsuki, Adv. Synth. Catal. 2002, 344, 131–147.
- 15With very low catalyst loading, the base-to-catalyst ratio of 10:1 implied a very low concentration of base, which gave irreproducible results. Hence, a larger amount of base was used.
- 16No reaction was observed in the complete absence of alcohol.
- 17With 5 mol % of base vs. substrate 3 a; see the Supporting Information.
- 18Running the hydrogenation reaction (almost) without solvent allows a more efficient use of reactors (higher process productivity) and is an important feature in the fine chemical industry.
- 19[RuCl2{(S)-tolBinap}{(R,R)-dpen}] gave 4 h with 94 % ee; see: T. Ohkuma, T. Hattori, H. Ooka, T. Inoue, R. Noyori, Org. Lett. 2004, 6, 2681–2683.
- 20aAlcohol 4 g was isolated in 92 % yield and 85 % ee from 3 g (0.4 mol) using a 3 g/EtONa/2 a ratio of 18700:100:1 in iPrOH. The synthetic sandalwood ingredient 4 m was isolated in 96 % yield from 3 m (5.27 mol) using a 3 m/NaOH/2 a ratio of 29300:1083:1 in MeOH;
- 20bunder conditions described in Table 2, catalyst 2 a gave 4 g in quantitative yield and 86 % ee. See the Supporting Information for further experimental details.
- 21C. Saudan, L. Saudan, WO 2010/038209, 2010.
- 22See the Supporting Information.
- 23TOF is defined as mol of the product per mol of catalyst per second.
- 24PNNP=(R,R)-bis[-2-(diphenylphosphino)benzylidene]cyclohexane-1,2-diamine. For the determination of TOF, see the Supporting Information.
- 25As thioethers can dealkylate upon coordination to a transition metal (S. G. Murray, F. R. Hartley, Chem. Rev. 1981, 81, 365, and references therein) or upon treatment with strong bases ( M. Gargir, Y. Ben-David, G. Leitus, Y. Diskin-Posner, L. J. W. Shimon, D. Milstein, Organometallics 2012, 31, 6207–6214) to generate thiolato complexes, we cannot exclude that this transformation is relevant to the catalyst activation or deactivation pathway.