Palladium-Catalyzed Reductive Carbonylation of Aryl Halides with N-Formylsaccharin as a CO Source†
Dr. Tsuyoshi Ueda
School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526 (Japan)
Process Technology Research Laboratories, Pharmaceutical Technology Division, Daiichi Sankyo Co., Ltd. 1-12-1 Shinomiya, Hiratsuka, Kanagawa 254-0014 (Japan)
Search for more papers by this authorDr. Hideyuki Konishi
School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Kei Manabe
School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526 (Japan)
School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526 (Japan)Search for more papers by this authorDr. Tsuyoshi Ueda
School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526 (Japan)
Process Technology Research Laboratories, Pharmaceutical Technology Division, Daiichi Sankyo Co., Ltd. 1-12-1 Shinomiya, Hiratsuka, Kanagawa 254-0014 (Japan)
Search for more papers by this authorDr. Hideyuki Konishi
School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Kei Manabe
School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526 (Japan)
School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526 (Japan)Search for more papers by this authorThis research was supported by the Daiichi Sankyo Co., Ltd.
Graphical Abstract
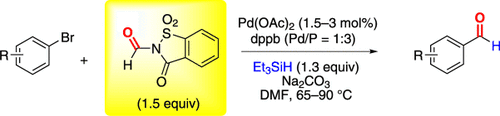
Easy peasy: The title reaction employs N-formylsaccharin, which is an easily accessible crystalline compound, as an effective CO source. The reactions proceed with a small excess of the CO source at moderate temperatures and were successfully applied to a wide range of aryl bromides. DMF=N,N-dimethylformamide, dppb=1,4-bis-(diphenylphosphino)butane.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303926_sm_miscellaneous_information.pdf2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. C. Larock, Comprehensive Organic Transformation: A Guide to Functional Group Preparation, 2nd ed., Wiley-VCH, New York, 1999.
- 2A. Schoenberg, R. F. Heck, J. Am. Chem. Soc. 1974, 96, 7761.
- 3
- 3aY. Misumi, Y. Ishii, M. Hidai, Organometallics 1995, 14, 1770;
- 3bH. Kotsuki, P. K. Datta, H. Suenaga, Synthesis 1996, 470;
- 3cL. Ashfield, C. F. J. Bernard, Org. Process Res. Dev. 2007, 11, 39.
- 4For a review on palladium-catalyzed carbonylation of aryl halides and related compounds, see: A. Brennführer, H. Neumann, M. Beller, Angew. Chem. 2009, 121, 4176;
10.1002/ange.200900013 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4114.
- 5
- 5aS. Klaus, H. Neumann, A. Zapf, D. Strübing, S. Hübner, J. Almena, T. Riermeier, P. Groβ, M. Sarich, W.-R. Krahnert, K. Rossen, M. Beller, Angew. Chem. 2006, 118, 161;
10.1002/ange.200502697 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 154;
- 5bH. Neumann, R. Kadyrov, X.-F. Wu, M. Beller, Chem. Asian J. 2012, 7, 2213;
- 5cA. G. Sergeev, A. Spannenberg, M. Beller, J. Am. Chem. Soc. 2008, 130, 15549.
- 6
- 6aFor a review on carbonylation without CO gas, see: T. Morimoto, K. Kakiuchi, Angew. Chem. 2004, 116, 5698;
10.1002/ange.200301736 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5580;
- 6bFor a review on [Mo(CO)6]-mediated carbonylation, see: L. R. Odell, F. Russo, M. Larhed, Synlett 2012, 685; Recent examples of uses of CO surrogates:
- 6cH. W. Lee, A. S. C. Chan, F. Y. Kwong, Chem. Commun. 2007, 2633;
- 6dP. Hermange, A. T. Lindhardt, R. H. Taaning, K. Bjerglund, D. Lupp, T. Skrydstrup, J. Am. Chem. Soc. 2011, 133, 6061;
- 6eS. D. Friis, R. H. Taaning, A. T. Lindhardt, T. Skrydstrup, J. Am. Chem. Soc. 2011, 133, 18114;
- 6fT. Morimoto, K. Yamasaki, A. Hirano, K. Tsutsumi, N. Kagawa, K. Kakiuchi, Y. Harada, Y. Fukumoto, N. Chatani, T. Nishioka, Org. Lett. 2009, 11, 1777.
- 7S. Cacchi, G. Fabrizi, A. Goggiamani, Org. Lett. 2003, 5, 4269.
- 8For recent examples of hydroxycarbonylation, see:
- 8aS. Korsager, R. H. Taaning, T. Skrydstrup, J. Am. Chem. Soc. 2013, 135, 2891;
- 8bP. Berger, A. Bessmernykh, J. Caille, S. Mignonac, Synthesis 2006, 3106.
- 9
- 9aT. Schareina, A. Zapf, A. Cotte, M. Gotta, M. Beller, Adv. Synth. Catal. 2010, 352, 1205;
- 9bS. Ko, C. Lee, M. G. Choi, Y. Na, S. Chang, J. Org. Chem. 2003, 68, 1607;
- 9cJ. F. Carpentier, Y. Castanet, J. Brocard, A. Mortreux, F. Petit, Tetrahedron Lett. 1991, 32, 4705.
- 10
- 10aT. Ueda, H. Konishi, K. Manabe, Org. Lett. 2012, 14, 3100;
- 10bT. Ueda, H. Konishi, K. Manabe, Tetrahedron Lett. 2012, 53, 5171;
- 10cT. Ueda, H. Konishi, K. Manabe, Org. Lett. 2012, 14, 5370;
- 10dTsuji et al. also reported the palladium-catalyzed esterification of aryl halides using aryl formates without CO. See: T. Fujihara, T. Hosoki, Y. Katafuchi, T. Iwai, J. Terao, Y. Tsuji, Chem. Commun. 2012, 48, 8012.
- 11For a review on metal-catalyzed aminocarbonylation, see: S. Roy, S. Roy, G. W. Gribble, Tetrahedron 2012, 68, 9867.
- 12S. Cacchi, G. Fabrizi, A. Goggiamani, J. Comb. Chem. 2004, 6, 692.
- 13During the publication of this work, a study appeared on reductive carbonylation with an acyl chloride as a CO surrogate. S. Korsager, R. H. Taaning, A. T. Lindhardt, T. Skrydstrup, J. Org. Chem. 2013, DOI: .
- 14Buchwald et al. used Pd/xantphos for alkoxycarbonylation of aryl bromides under a CO atmosphere. See: J. R. Martinelli, D. A. Watson, D. M. M. Freckmann, T. E. Barder, S. L. Buchwald, J. Org. Chem. 2008, 73, 7102.
- 15
- 15aT. Cochet, V. Bellosta, A. Greiner, D. Roche, J. Cossy, Synlett 2011, 1920;
- 15bN-Formylsaccharin is commercially available from Tokyo Chemical Industry Co., Ltd. (TCI).
- 16I. Koppel, J. Koppel, I. Leito, V. Pihl, L. Grahn, U. Ragnarsson, J. Chem. Res. 1994, 6, 1173.
- 17
- 17aJ. R. Martinelli, T. P. Clark, D. A. Watson, R. H. Munday, S. L. Buchwald, Angew. Chem. 2007, 119, 8612;
10.1002/ange.200702943 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8460;
- 17bR. H. Munday, J. R. Martinelli, S. L. Buchwald, J. Am. Chem. Soc. 2008, 130, 2754.
- 18Cy=cyclohexyl, o-Tol=2-methylphenyl, dppm=bis(diphenyl-phosphino)methane, dppe=1,2-bis(diphenylphosphino)ethane, dppp=1,3-bis(diphenylphosphino)propane, dppb=1,4-bis-(diphenylphosphino)butane, dpppe=1,5-bis(diphenylphosphino)-pentane, dppf=1,1′-bis(diphenylphosphino)ferrocene, dppBz=1,2-bis(diphenylphosphino)benzene, DCyPP=1,3-bis(dicyclohexylphosphino)propane, DCyPB=1,4-bis(dicyclohexylphosphino)butane, dpephos=bis(2-(2-diphenylphosphino)phenyl) ether, xantphos=4,5-bis(diphenylphosphino)-9,9-dimethylxanthene, binap=bis(diphenylphosphino)-1,1′-binaphthyl, Dt-bpe=1,1′-bis(di-tert-butylphosphino)ferrocene.
- 19Other silanes, including PhMe2SiH, Ph2MeSiH, Ph3SiH, polymethylhydrosiloxane, iPr3SiH, and (EtO)3SiH were tested in the reaction of 1 k, but only gave poor yields of 2 k (3–50 %).
- 20The preparation of 6 was performed according to: T. Imai, T. Okunoyama, M. Okoshi, J. Chem. Soc. Jpn. 1975, 123. See the Supporting Information for details.