Selective Nitrate Binding in Competitive Hydrogen Bonding Solvents: Do Anion–π Interactions Facilitate Nitrate Selectivity?†
Michelle M. Watt
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorDr. Lev N. Zakharov
CAMCOR—Center for Advanced Materials Characterization in Oregon, University of Oregon, Eugene, OR 97403-1443 (USA)
Search for more papers by this authorCorresponding Author
Prof. Michael M. Haley
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)Search for more papers by this authorCorresponding Author
Prof. Darren W. Johnson
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)Search for more papers by this authorMichelle M. Watt
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorDr. Lev N. Zakharov
CAMCOR—Center for Advanced Materials Characterization in Oregon, University of Oregon, Eugene, OR 97403-1443 (USA)
Search for more papers by this authorCorresponding Author
Prof. Michael M. Haley
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)Search for more papers by this authorCorresponding Author
Prof. Darren W. Johnson
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)Search for more papers by this authorThis work was supported by NIH grant R01-GM087398, which also funded early stage intellectual property that was licensed by SupraSensor Technologies, a company co-founded by the principal investigators. We also thank the National Science Foundation for support in the form of an instrumentation grant (CHE-0923589) and a GK12 fellowship to M.M.W. (DGE-0742540). The authors acknowledge the Biomolecular Mass Spectrometry Core of the Environmental Health Sciences Core Center at Oregon State University (NIH P30ES000210). We thank Timothy E. Robitshek for assistance in the synthesis of compound 2.
Graphical Abstract
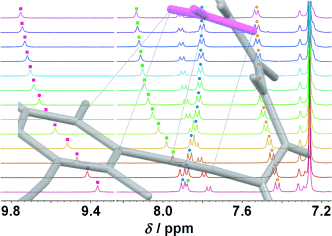
New tripodal urea receptors demonstrate preferential binding of anions over competitive hydrogen bonding solvents. 1H NMR titrations in 10 % [D6]DMSO/CDCl3 show a higher affinity for nitrate over the halides for the fluorinated receptor, which is lost when the fluorine atoms are absent. An anion–π interaction between the nitrate and the π-system of the ethynyl-substituted arene is proposed as the source of this selectivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303881_sm_miscellaneous_information.pdf2.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Işiklan, M. A. Saeed, A. Pramanik, B. M. Wong, F. R. Fronczek, M. A. Hossain, Cryst. Growth Des. 2011, 11, 959–963;
- 1bS. J. Brooks, P. A. Gale, M. E. Light, Chem. Commun. 2006, 4344–4346;
- 1cP. V. Santacroce, O. A. Okunola, P. Y. Zavalij, J. T. Davis, Chem. Commun. 2006, 3246–3248;
- 1dP. Tongraung, N. Chantarasiri, T. Tuntulani, Tetrahedron Lett. 2003, 44, 29–32;
- 1eR. Herges, A. Dikmans, U. Jana, F. Köhler, P. G. Jones, I. Dix, T. Fricke, B. König, Eur. J. Org. Chem. 2002, 3004–3014;
- 1fF. Hettche, P. Beiss, R. W. Hoffmann, Chem. Eur. J. 2002, 8, 4946–4956;
10.1002/1521-3765(20021104)8:21<4946::AID-CHEM4946>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 1gA. P. Bisson, V. M. Lynch, M. K. C. Monahan, E. V. Anslyn, Angew. Chem. 1997, 109, 2435–2437;
10.1002/ange.19971092115 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2340–2342.
- 2
- 2aD. Makuc, M. Albrecht, J. Plavec, K. Rissanen, A. Valkonen, C. A. Schalley, Eur. J. Org. Chem. 2009, 4854–4866;
- 2bJ. L. Sessler, D. An, W.-S. Cho, V. Lynch, M. Marquez, Chem. Eur. J. 2005, 11, 2001–2011;
- 2cD. H. Burns, K. Calderon-Kawasaki, S. Kularatne, J. Org. Chem. 2005, 70, 2803–2807;
- 2dJ. P. Clare, A. J. Ayling, J.-B. Joos, A. L. Sisson, G. Magro, M. N. Pérez-Payán, T. N. Lambert, R. Shukla, B. D. Smith, A. P. Davis, J. Am. Chem. Soc. 2005, 127, 10739–10746.
- 3
- 3aO. Hudeček, J. Budka, H. Dvořáková, P. Cuřínová, I. Císařová, P. Lhoták, New J. Chem. 2013, 37, 220–227;
- 3bP. G. Young, K. A. Jolliffe, Org. Biomol. Chem. 2012, 10, 2664–2672;
- 3cH. Juwarker, J. M. Lenhardt, J. C. Castillo, E. Zhao, S. Krishnamurthy, R. M. Jamiolkowski, K.-H. Kim, S. L. Craig, J. Org. Chem. 2009, 74, 8924–8934;
- 3dP. Byrne, D. R. Turner, G. O. Lloyd, N. Clarke, J. W. Steed, Cryst. Growth Des. 2008, 8, 3335–3344;
- 3eK.-J. Chang, D. Moon, M. S. Lah, K.-S. Jeong, Angew. Chem. 2005, 117, 8140–8143; Angew. Chem. Int. Ed. 2005, 44, 7926–7929.
- 4
- 4aK. Choi, A. D. Hamilton, J. Am. Chem. Soc. 2003, 125, 10241–10249;
- 4bK. Choi, A. D. Hamilton, J. Am. Chem. Soc. 2001, 123, 2456–2457.
- 5R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley, S. Matile, Nat. Chem. 2010, 2, 533–538.
- 6A. Vargas Jentzsch, D. Emery, J. Mareda, P. Metrangolo, G. Resnati, S. Matile, Angew. Chem. 2011, 123, 11879–11882;
10.1002/ange.201104966 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11675–11678.
- 7G. Gil-Ramírez, E. C. Escudero-Adán, J. Benet-Buchholz, P. Ballester, Angew. Chem. 2008, 120, 4182–4186;
10.1002/ange.200800636 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4114–4118.
- 8L. Adriaenssens, C. Estarellas, A. V. Jentzsch, M. M. Belmonte, S. Matile, P. Ballester, J. Am. Chem. Soc. 2013, 135, 8324–8330.
- 9We recognize the observed interaction between nitrate and the π-system may simply be π-stacking between nitrate and an arylalkyne; however, such an interaction might still be broadly described as “anion–π”, as one partner in the association is anionic.
- 10
- 10aJ. M. Engle, C. N. Carroll, D. W. Johnson, M. M. Haley, Chem. Sci. 2012, 3, 1105–1110;
- 10bC. N. Carroll, B. A. Coombs, S. P. McClintock, C. A. Johnson II, O. B. Berryman, D. W. Johnson, M. M. Haley, Chem. Commun. 2011, 47, 5539–5541;
- 10cC. N. Carroll, J. J. Naleway, M. M. Haley, D. W. Johnson, Chem. Soc. Rev. 2010, 39, 3875–3888;
- 10dC. N. Carroll, O. B. Berryman, C. A. Johnson, L. N. Zakharov, M. M. Haley, D. W. Johnson, Chem. Commun. 2009, 2520–2522.
- 11
- 11aB. P. Hay, M. Gutowski, D. A. Dixon, J. Garza, R. Vargas, B. A. Moyer, J. Am. Chem. Soc. 2004, 126, 7925–7934;
- 11bB. P. Hay, D. A. Dixon, J. C. Bryan, B. A. Moyer, J. Am. Chem. Soc. 2002, 124, 182–183.
- 12G. Hennrich, A. M. Echavarren, Tetrahedron Lett. 2004, 45, 1147–1149.
- 13W. B. Wan, M. M. Haley, J. Org. Chem. 2001, 66, 3893–3901.
- 14
- 14aV. K. Chaikovskii, V. D. Filimonov, A. A. Funk, V. I. Skorokhodov, V. D. Ogorodnikov, Russ. J. Org. Chem. 2007, 43, 1291–1296;
- 14bS. Z. Vatsadze, I. D. Titanyuk, A. V. Chernikov, N. V. Zyk, Russ. Chem. Bull. 2004, 53, 471–473.
- 15P. Thordarson, Chem. Soc. Rev. 2011, 40, 1305–1323.
- 16Values obtained using the 1:1 Thordarson fitting program consistantly gave association constants on the order of 104 L mol−1 for bromide and 105 L mol−1 for nitrate, albeit with high errors and poor overall fit.
- 17Given the possibility of 2:1 binding modes for NO3− or Br− in [D6]acetone, and evidence of solid-state dimerization, self-association of 1 in acetone was investigated. The data obtained could not be fit to a self-association/dimerization model (even when dimerization was assumed). While this evidence provides little assistance in fitting NO3− or Br− titration data, it shows the receptor aggregates in acetone. This, coupled to the potential for association constants larger than 104 L mol−1, led us to investigate the binding in other solvents.
- 18W.-J. Chu, Y. Yang, C.-F. Chen, Org. Lett. 2010, 12, 3156–3159.
- 19No changes in chemical shift were observed upon titration of nitrate into 1 in neat [D6]DMSO.
- 20Results indicate a 2:1 binding model breaking up to 1:1 upon the further addition of anion. The 2:1 model likely results from self-aggregation of the receptor in these polar solvent mixtures.
- 21Statistical significance determined by a t-test comparing the values obtained for nitrate binding versus those of chloride binding. The difference between the two was found to be significant with 99.9 % certainty.
- 22The authors would like to thank an anonymous reviewer for describing this interaction as a “π–π-assisted anion–π” interaction, which we think aptly describes such an attraction.
- 23
- 23aB. W. Tresca, L. N. Zakharov, C. N. Carroll, D. W. Johnson, M. M. Haley, Chem. Commun. 2013, 49, 7240–7242;
- 23bJ. V. Gavette, N. S. Mills, L. N. Zakharov, C. A. Johnson II, D. W. Johnson, M. M. Haley, Angew. Chem. 2013, 125, 10460–10464; Angew. Chem. Int. Ed. 2013, 52, 10270–10274.